
Robotic distal pancreatectomy with celiac axis resection for locally advanced pancreatic cancer
Introduction
Surgery provides the only opportunity for patients with pancreatic adenocarcinoma (PDAC) to achieve long-term survival. However, due to a combination of aggressive tumor biology and the anatomic location of the pancreas, only 20% of patients present with resectable disease at the time of diagnosis. Neoadjuvant chemotherapy with or without radiation therapy has been used to downstage patients and facilitate surgical resection with curative intent in patients with locally advanced tumors (1). Such multimodal therapy has been used with success in select patients to increase the chances of an R0 resection and treat regional lymph node basins (2). This approach when followed by adjuvant chemotherapy can result in improved survival (3).
With improved outcomes following the addition of neoadjuvant therapy and increased experience with pancreatectomy, surgeons have become more aggressive in patients with locally advanced tumors with vascular involvement (4). In 1953, Lyon Appleby first described en bloc resection of the celiac trunk, total gastrectomy, and distal pancreatectomy (DP) as an approach to patients with locally advanced gastric cancer (5). A variation of this approach has been used in patients with pancreatic body or tail tumors that invade the celiac axis. The modified Appleby operation preserves the stomach but includes distal pancreatectomy and splenectomy with en bloc celiac axis resection (DP-CAR) (4,6).
Using an aggressive surgical approach in a cancer with notoriously aggressive tumor biology requires a careful assessment of the risks and benefits. A recent analysis of the NSQIP database found that DP-CAR was associated with increased operative time, higher post-operative acute kidney injury, as well as higher 30-day mortality compared to DP alone (7). However, a more recent meta-analysis of 18 studies found no statistically significant differences in morbidity or mortality following DP-CAR compared to DP alone (8). In addition, the 1-, 2-, and 3-year survival rates were 62.2%, 30.2%, and 18.7% following DP-CAR and were similar to patients following DP. More so, those treated with DP-CAR had improved 1-year survival compared to patients who received palliative treatments (8). As such, the survival advantage of an aggressive surgical approach relies heavily on minimizing operative morbidity and mortality.
Outcomes of robotic DP-CAR: University of Pittsburgh Experience
Efforts to improve the safety of pancreatectomy have led to the development and implementation of minimally invasive and robotic approaches to both pancreatoduodenectomy and segmental pancreatectomy (9,10). As our experience at the University of Pittsburgh has grown, the complexity of cases has increased. We have reported the operative outcomes of 30 DP-CARs (11). Twenty-eight patients had PDAC, and of these, all but one (96%) received neoadjuvant therapy. Nineteen were completed robotically without the need for conversion to open. This included four patients who required a concomitant tangential venous resection that was able to be performed. We found that robotic DP-CAR had comparable morbidity and mortality to the 11 that were performed in open fashion, however the robotic approach was associated with a statistically significant (P<0.05) reduction in operative time (316 vs. 476 min), intraoperative blood loss (393 vs. 1,736 mL), and blood transfusion rate (0% vs. 54%) (11). The incidence of post-operative pancreatic fistula (POPF), Grade B/C POPF, and serious morbidity (Clavien-Dindo grade 3–4) were comparable between the two cohorts. The advantages for the robotic approach in this series may have been related to selection bias, since the robotic cases were performed after the learning curve with the open approach was reached. Importantly, both groups had similar lengths of stay, readmission rates, and receipt of adjuvant therapy (11). The median survival was nearly three years for the entire cohort and was comparable amongst the two approaches. In our experience, robotic DP-CAR is safe and effective and may improve survival in carefully selected patients.
Patient selection
The importance of patient selection for DP-CAR cannot be overstated. Anatomic, tumor specific, and patient factors are important in determining resectability. There are four criteria that a patient must meet to be considered an operative candidate at our institution. These have been published previously and include: (I) tumor of the body/tail of the pancreas with involvement of any branch(es) of the celiac axis, but not of the celiac trunk itself; (II) the gastroduodenal artery (GDA) must be preserved and without tumor involvement; (III) treatment with neoadjuvant chemotherapy (with or without radiation) to treat micro metastatic disease and allow for assessment of tumor biology prerequisite; (IV) good performance status (12).
Operative approach
Our robotic approach to DP-CAR has been published previously (12). Following port placement (Figure 1) a careful exploration of the abdomen is performed. Entrance into the lesser sac is achieved after dividing the omentum from the transverse colon. The stomach is retracted allowing for traction to be placed on the left gastric artery and vein. The common hepatic artery (CHA) is identified and traced distally until the takeoff of the GDA. To ensure the GDA is able to provide collateral flow to the liver after sacrificing the celiac trunk, we clamp the CHA and perform a laparoscopic duplex ultrasound of the GDA. After confirming triphasic perfusion at the porta hepatis, the pancreatic neck is dissected from the retroperitoneum and transected using a laparoscopic stapler (Figure 2A). Next, the splenic vein is identified and transected at the insertion with the superior mesenteric vein/portal vein confluence. Attention is then turned towards transection of the splenic attachments and the spleen and tail of the pancreas are mobilized from lateral to medial with the retroperitoneal fascia until left lateral wall of the celiac axis is encountered.
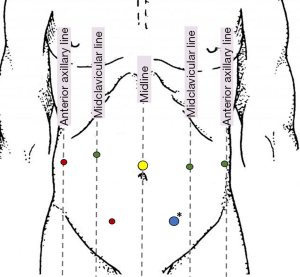
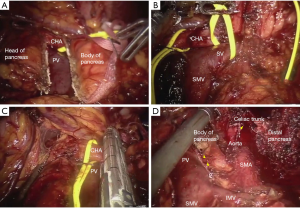
After dissection of the body/tail of the pancreas and spleen, we then proceed with the dissection of the celiac axis superiorly. The CHA is identified and transected just proximal to the GDA (Figure 2B,C). Next, we identify the left gastric artery and vein and divide these with a vascular stapler. The supra celiac aorta is exposed by transecting the crural fibers, and the aorta is followed inferiorly to reach the celiac trunk. The robotic hook cautery is used to expose the celiac trunk through division of the celiac plexus and surrounding lymphatics. Once properly exposed, the celiac axis is transected using a laparoscopic vascular stapler (Figure 2D). The specimen is placed in an Endo Catch specimen pouch and removed after enlarging the 12 mm port in the left lower quadrant (Figure 1). After ensuring adequate hemostasis, the pancreatic bed is drained and 12 mm port sites closed.
Conclusions
The robotic approach to DP-CAR is safe, with comparable morbidity and mortality to an open approach once the learning curve for open DP-CAR and robotic surgery are reached. Data on the robotic approach is scarce, however based on our experience, use of the robotic platform may be associated with reductions in operative time, intraoperative blood-loss and transfusion rate. While some patients may benefit, the importance of appropriate patient selection cannot be overstated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jin He) for the series “Robotic Surgery for Pancreatic Cancer” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2018.01.07). The series “Robotic Surgery for Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peters NA, Javed AA, Cameron JL, et al. Modified Appleby Procedure for Pancreatic Adenocarcinoma: Does Improved Neoadjuvant Therapy Warrant Such an Aggressive Approach? Ann Surg Oncol 2016;23:3757-64. [Crossref] [PubMed]
- Petrelli F, Coinu A, Borgonovo K, et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 2015;44:515-21. [Crossref] [PubMed]
- Tang K, Lu W, Qin W, et al. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. Pancreatology 2016;16:28-37. [Crossref] [PubMed]
- Ozaki H, Kinoshita T, Kosuge T, et al. An aggressive therapeutic approach to carcinoma of the body and tail of the pancreas. Cancer 1996;77:2240-5. [Crossref] [PubMed]
- Appleby LH. The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer 1953;6:704-7. [Crossref] [PubMed]
- Mayumi T, Nimura Y, Kamiya J, et al. Distal pancreatectomy with en bloc resection of the celiac artery for carcinoma of the body and tail of the pancreas. Int J Pancreatol 1997;22:15-21. [Crossref] [PubMed]
- Beane JD, House MG, Pitt SC, et al. Distal pancreatectomy with celiac axis resection: what are the added risks? HPB (Oxford) 2015;17:777-84. [Crossref] [PubMed]
- Gong H, Ma R, Gong J, et al. Distal Pancreatectomy With En Bloc Celiac Axis Resection for Locally Advanced Pancreatic Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2016;95:e3061 [Crossref] [PubMed]
- Boggi U, Amorese G, Vistoli F, et al. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc 2015;29:9-23. [Crossref] [PubMed]
- Kendrick ML, van Hilst J, Boggi U, et al. Minimally invasive pancreatoduodenectomy. HPB (Oxford) 2017;19:215-24. [Crossref] [PubMed]
- Ocuin LM, Miller-Ocuin JL, Novak SM, et al. Robotic and open distal pancreatectomy with celiac axis resection for locally advanced pancreatic body tumors: a single institutional assessment of perioperative outcomes and survival. HPB (Oxford) 2016;18:835-42. [Crossref] [PubMed]
- Greer J, Zureikat AH. Robotic distal pancreatectomy combined with celiac axis resection. J Vis Surg 2017;3:145. [Crossref] [PubMed]
Cite this article as: Beane JD, Zureikat AH. Robotic distal pancreatectomy with celiac axis resection for locally advanced pancreatic cancer. Ann Pancreat Cancer 2018;1:11.

