
Robotic total pancreatectomy en masse without division of the pancreatic neck
Introduction
Neuroendocrine tumors (NETs) are epithelial neoplasms with neuroendocrine differentiation that can arise in a variety of organs. NET that arise in the pancreas (PanNETs) comprise less than 3% of all pancreatic neoplasms (1). The most recent classification by the World Health Organization classifies NET based on the degree of differentiation (well vs. poorly), tumor grade, mitotic count, and Ki-67 index (2). Likely due to increased cross-sectional imaging, there has been an increasing incidence of these tumors.
Though a robotic operation is now considered a standard approach for various general, urological, and gynecological procedures, utilization of robotic surgery for complex pancreatic resections remains low (3). In fact, the majority of pancreatic resections in the US are performed in a traditional open manner despite studies showing robotic approach to be equally safe to an open approach (3,4). This low utilization is undoubtedly multifactorial and may be largely related to the complexity of the pancreatic resection and the absence of an adequate number of structured robotic training programs throughout the country (5-10). Common contraindications for robotic pancreatectomy include difficult access to abdominal cavity due to severe intraabdominal adhesions from previous surgeries or peripancreatic inflammation, intolerance of pneumoperitoneum due to cardiopulmonary dysfunction, difficult anatomy due to involvement of major vessels around the pancreas, and inadequate robotic skills. Despite this, there are few contraindications to a robotic approach to pancreatic resections. Here we review a robotic total pancreatectomy en masse without division of the pancreatic neck for a large NET that occupied the pancreatic head, neck, and body.
Case presentation
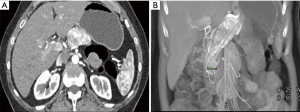
A 56-year-old female presented to our surgical clinic with a large biopsy-proven NET that was found after cross-sectional imaging for abdominal pain. On imaging, the patient had a PanNET that infiltrated the pancreatic head, neck, and body (Figure 1) and measured approximately 9 cm in length. Given the size and symptomatology of this tumor, the patient was offered a robotic total pancreatectomy en masse without division of the pancreatic neck in order to prevent any inadvertent tumor spillage in the abdomen.
Surgical technique
A comprehensive robotic surgical team is required to effectively perform complex robotic operations and this team includes an anesthesiologist, nursing staff familiar with the robotic instruments and setup, an operating console surgeon, and a bedside surgical assistant for port-placement, instrument exchange, and perhaps most importantly, retraction and suctioning. Both the bedside scrub nurse and room circulator should be proficient at robotic operations to facilitate efficient instrument setup and exchange.
The patient was positioned in a supine position with legs spread on a split-leg operating table and arms out at 90 degrees. Peripheral intravenous access, an arterial monitoring line, and a Foley catheter were placed as well as a nasogastric tube for decompression of the stomach. Monitors were placed over both the left and right shoulders of the patient to allow adequate view for the surgical assistant and scrub technician. The first assistant stands between the legs of the patient while the scrub nurse stands to the left of the patient. The abdomen was entered using the Veress technique through a supraumbilical incision. Upon inspection of the abdomen, no metastatic disease was appreciated. Four additional robotic 8 mm ports were placed in a straight line across the mid abdomen under direct visualization. The robot (DaVinci Xi system) was then docked from the patient’s left side.
Exposure and dissection
The dissection begins at the hepatic flexure and the right colon was carefully dissected away from the liver. The ligamentum teres was dissected from the abdominal wall and encircled using an endostitch. A stab incision was then made in the right sub-xyphoid area and the endostitch was pulled through the abdominal wall and secured in order to expose the porta hepatis. A cholecystectomy was then performed by dissecting Callot’s triangle using the hook cautery. The cystic duct was identified, clipped, and transected. If needed for exposure, a stitch can be placed through the infundibulum of the gallbladder and the suture can be pulled percutaneously to provide cephalad retraction in a similar fashion to the retraction of the ligament teres. The common bile duct was then transected and a clip was placed on the proximal duct to prevent ongoing spillage of bile in the abdomen during the remainder of the operation.
The porta hepatis was carefully dissected using the hook cautery until the common hepatic, proper hepatic, and gastroduodenal arteries were carefully identified. The gastroduodenal artery was test clamped to confirm adequate blood flow to the proper hepatic artery via the common hepatic artery. The gastroduodenal artery was sequentially tied using a 0-silk suture and Hem-o-lok clips, and then transected. The stomach was carefully dissected around the pylorus and divided using an endo-GIA stapler. Once this was performed, the lesser sac was entered by dividing the gastrocolic ligament along the greater curvature of the stomach using the robotic vessel sealer device. The stomach was retracted cephalad to expose the anterior surface of the pancreas. A Kocher maneuver was performed and the ligament of Treitz was divided from the right side of the abdomen. Once transected, the mobilized jejunum was brought through the ligament of Treitz defect and divided at approximately 20 cm downstream with an endo-GIA stapler.
Attention was turned to the inferior border of the pancreas. Sharp dissection was performed using a combination of hook cautery and the vessel sealer device. Careful dissection was performed on the inferior margin of the pancreas until the superior mesenteric vein was visualized. Once this was visualized, an infra-pancreatic tunnel was bluntly dissected under direct visual guidance. Using a long tip-up blunt grasper, an umbilical tape was passed underneath the neck of the pancreas and used to retract the pancreas in a superior/lateral direction (Figure 2). The use of the umbilical tape to provide anterior retraction was a key step of the operation as it provided adequate visualization and exposure of the superior mesenteric vein (SMV) and uncinate process without division of the pancreatic neck.
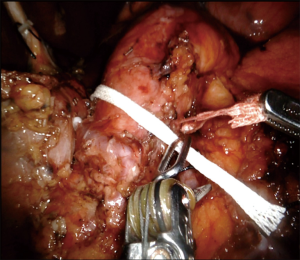
The splenic artery was then dissected from the superior border of the pancreas. Once identified and dissected cleanly, this artery was tied using a 0-silk tie and double clippled with Hem-o-lok and then transected sharply using robotic scissors. The splenic vein was then identified through further dissection of the inferior border of the pancreas in combination with cephalad/superior retraction of the pancreas using the umbilical tape. The splenic vein was similarly tied, clipped, and transected.
Attention was then turned to the uncinate process. In cases where the neck of the pancreas was not divided due to tumor involvement, retraction of the pancreas anterior and lateral was performed using the umbilical tape and the 3rd robotic arm. The uncinate process was transected using a combination of the harmonic scalpel, the vessel sealer, and monopolar cautery. Individual branches to the SMV are individually ligated as necessary.
The inferior and superior border of the pancreas were then dissected more in a median to lateral fashion. The short gastric vessels were transected, and the spleens were mobilized. The specimen was then placed in an endocatch bag along with the gallbladder and extracted through a 6 cm Pfannenstiel incision.
Reconstruction
Reconstruction began with the hepaticojejunostomy. A small enterotomy was made in the jejunum which was brought up through the ligament of Treitz defect. The size of the enterotomy matched the size of the hepatic duct. A 5-0 PDS suture with an RB1 needle was placed at the corner and tied. The posterior row was then completed using 5-0 PDS sutures in an interrupted fashion. The posterior row may be completed in a running fashion depending on the size of the hepatic duct. A pediatric feeding tube can be placed as a stent to aid in reconstruction of the anterior layer of this hepaticojejunostomy. The anterior row of the hepaticojejunostomy was completed in a similar fashion using interrupted 5-0 PDS sutures.
The gastrojejunostomy was performed by bringing the jejunum in an antecolic side-to-side fashion. A traction suture was placed in the jejunum and stomach and used to aid in stapler placement. A jejunotomy and gastrotomy were created using the harmonic scalpel approximately 3 cm away from the gastric staple line. The anastomosis was performed using an endo-GIA stapler. The common enterotomy was then closed in two lawyers using a running 3-0 V-Loc™ suture.
The abdomen was then thoroughly irrigated and one drain was placed through the left port site. This drain was placed in the splenic fossa and travels across the pancreatic bed, terminating below the hepaticojejunostomy anastomosis. The abdomen was desufflated and the assistant 10 mm port was closed using an interrupted #1 PDS suture.
Patient outcome
Following the operation, the patient remained in the hospital for a total length of stay of 7 days mainly for glucose control and diabetes teaching. There were no immediate postoperative complications. Final pathology revealed a 9×3×2.5 centimeter well differentiated NET with negative margins and negative nodal involvement.
Rationale
Though pancreatic NET are a relatively rare group of neoplasms, long-term prognosis varies due to the wide-ranging grade and metastatic potential found among these group of these tumors (11). As such, attention to strict oncological principles during surgical extirpation of these tumors is important. As a relatively rare tumor, implications of tumor capsule violation and tumor spillage has not been previously reported in patients with pancreatic NETs. However, tumor spillage during surgical resection of various other tumors has been shown to be an independent risk factor for recurrence and poor long-term outcomes (12-15). In the case presented, division of the pancreatic neck during total pancreatectomy would have violated the tumor capsule and caused inadvertent tumor spillage in the abdomen. As such, total pancreatectomy without division of the pancreatic neck is important to prevent this occurrence and is possible during a robotic approach.
The use of a robotic approach for total pancreatectomy has previously been reported. In a report from 2010, Giulianotti et al. reported on the safety and feasibility of robotic total pancreatectomy using the da Vinci robotic system (16). To our knowledge, however, this is the first report of a total pancreatectomy en masse without division of the pancreatic neck for a large tumor spanning the near entirety of the pancreas. This report shows that a robotic approach is safe and feasible and particularly helpful in cases when division of the pancreatic neck may cause inadvertent tumor spillage.
Conclusions
In conclusion, robotic total pancreatectomy en masse without division of the pancreatic neck can be performed safely in instances where a large tumor was traversing longitudinally along the pancreas without involvement of major vessels. Tumor size and the level of pancreatic involvement are not contraindications to a robotic approach. Meticulous dissection and adequate exposure are key steps to perform this operation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Pancreatic Cancer for the series “Robotic Surgery for Pancreatic Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2018.02.02). The series “Robotic Surgery for Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. JH served as the unpaid Guest Editor of the series and serves as an unpaid section editor of Annals of Pancreatic Cancer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Halfdanarson TR, Rubin J, Farnell MB, et al. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 2008;15:409-27. [Crossref] [PubMed]
- Ejaz A, Reames BN, Maithel S, et al. The impact of extrahepatic disease among patients undergoing liver-directed therapy for neuroendocrine liver metastasis. J Surg Oncol 2017;116:841-7. [Crossref] [PubMed]
- Ejaz A, Sachs T, He J, et al. A comparison of open and minimally invasive surgery for hepatic and pancreatic resections using the Nationwide Inpatient Sample. Surgery 2014;156:538-47. [Crossref] [PubMed]
- Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. [Crossref] [PubMed]
- Stauffer JA, Asbun HJ. Minimally invasive pancreatic surgery. Semin Oncol 2015;42:123-33. [Crossref] [PubMed]
- Matsuoka L, Parekh D. The minimally invasive approach to surgical management of pancreatic diseases. Gastroenterol Clin North Am 2012;41:77-101. [Crossref] [PubMed]
- Kendrick ML. Laparoscopic and robotic resection for pancreatic cancer. Cancer J 2012;18:571-6. [Crossref] [PubMed]
- Wang Y, Bergman S, Piedimonte S, et al. Bridging the gap between open and minimally invasive pancreaticoduodenectomy: the hybrid approach. Can J Surg 2014;57:263-70. [Crossref] [PubMed]
- Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. [Crossref] [PubMed]
- Rosales-Velderrain A, Bowers SP, Goldberg RF, et al. National trends in resection of the distal pancreas. World J Gastroenterol 2012;18:4342-9. [Crossref] [PubMed]
- Gorelik M, Ahmad M, Grossman D, et al. Nonfunctioning Incidental Pancreatic Neuroendocrine Tumors: Who, When, and How to Treat? Surg Clin North Am 2018;98:157-67. [Crossref] [PubMed]
- Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg 1999;229:292-7. [Crossref] [PubMed]
- Zirngibl H, Husemann B, Hermanek P. Intraoperative spillage of tumor cells in surgery for rectal cancer. Dis Colon Rectum 1990;33:610-4. [Crossref] [PubMed]
- Pidhorecky I, Cheney RT, Kraybill WG, et al. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 2000;7:705-12. [Crossref] [PubMed]
- Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006;243:738-45; discussion 745-7. [Crossref] [PubMed]
- Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. [Crossref] [PubMed]
Cite this article as: Ejaz A, Espin L, He J. Robotic total pancreatectomy en masse without division of the pancreatic neck. Ann Pancreat Cancer 2018;1:14.

