Robotic distal pancreatectomy: experience in a high-volume center
Introduction
Pancreatic surgery is one of the most challenging abdominal operations with high perioperative and mortality rates (1,2). Recent developments in robotic instruments and techniques can overcomes many inherent limitations of laparoscopic surgery (3). Three-dimensional visualization, improved dexterity, and filtration of natural tremor have been integrated to made robotic pancreatic surgery to become widely accepted by surgeons (4-6). As robotic distal pancreatectomy (RDP) only requires limited dissection around splenic vessels and it does not require any reconstruction, it has been broadly applied in pancreatic centers (7,8). In this study, we evaluated our experience on the safety and feasibility of RDP in a high-volume robotic center in China.
Methods
We retrospectively analyzed the clinical data of consecutive 210 patients who underwent RDP at the department of Hepatobiliary and Pancreatic Surgical Oncology, People’s Liberation Army General Hospital in China from November 2011 to August 2016. The study was approved by the Institutional Review Board of the People’s Liberation Army General Hospital (S2016-098-01).
Preoperative evaluation
A contrast-enhanced computed tomography (CT) scan or magnetic resonance imaging (MRI) was performed as a routine diagnostic procedure. When images were insufficient to diagnose, the patient was referred for endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) with a cystic fluid analysis to differentiate between serous or mucinous tumors.
Selection of the procedure
The inclusion criteria were: (I) presence of a resectable benign, malignant, or borderline malignant pathology of the pancreatic body and tail; (II) no general medical conditions that contraindicated anesthesia and surgery; and (III) no history of previous major upper abdominal surgery. The exclusion criteria were: (I) tumors larger than 10 cm; and (II) pancreatic adenocarcinoma (PDAC) with metastases.
All included patients were informed of the advantages and disadvantages of robotic approach as well as the possible complications and costs. They made the decision to undergo RDP and gave written informed consents for the chosen operation and this study. All robotic operations were performed by the same surgical team.
Perioperative data
The baseline demographics, perioperative and pathology data were obtained from the electronic medical records. Operation time (OT), estimated blood loss (EBL), blood transfusion, rate of conversion to laparotomy, splenic preservation (SP) rates, splenic vessel preservation (SVP) rates, tumor histopathology, postoperative complications, and postoperative hospital stay (PHS), were analyzed retrospectively. Readmission rates within 90 days and 30-day mortality rates were also examined. The postoperative complications were graded using the Clavien–Dindo classification (9). The grading system for a postoperative pancreatic fistula was based on the International Study Group of Pancreatic Fistula (grades A, B, and C) (10).
Surgical technique and follow up
All the robotic surgical procedures were performed by a single team of surgeons using the Da Vinci Si Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). This team had performed more than 1,000 robotic pancreatic surgeries.
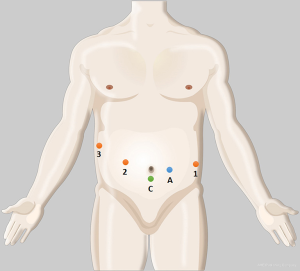
Under general anesthesia, the patient was placed in a supine decubitus position. Five ports (3, 8 mm; 2, 12 mm) were placed as shown in Figure 1. After docking, we divided the gastrocolic ligament and mobilized the distal pancreas by a coagulation hook or ultrasonic scalpel (Harmonic®, Ethicon Endo-Surgery, Cincinnati, OH, USA). After the superior mesenteric/portal vein (SMV/PV) is mobilized, DP is resected along the left margin of the SMV with an endoscopic linear staple (EC-60, Ethicon Endo-Surgery). For a splenic vessel-sacrificing operation or splenectomy, the splenic vessels are divided with a linear staple and white staple cartridge application. The feeding vessels were isolated and clipped by Hem-o-lok® clip (TFX Medical Ltd., RTP Durham, NC, USA) or ligatures with Prolene® (Ethicon, Sommerville, NJ, USA) sutures, and divided.
We try to preserve the spleen and splenic vessels when performing RDP in patients with benign and borderline malignant tumors. Excessive blood loss, major vessel invasion, and severe pancreatitis and longer surgical time, are common indications for converting to splenectomy or Warshaw procedure (11). We performed RDP for PDAC according to radical antegrade modular pancreatosplenectomy (RAMPS) approach (12).
All patients were followed up 1 month after discharge, 3 months in the first year and then at 6-month intervals thereafter.
Statistical analysis
Continuous data are presented as mean ± SD or median and interquartile range (IQR) according to their distributions. All statistical analyses were performed using the SPSS v22.0 software (SPSS, Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 210 patients underwent RDP during the study period. The patients included 74 men and 136 women with a mean age of 48.3 years. Most patients were asymptomatic (159, 75.7%), and 51 patients (24.3%) presented with abdominal pain. The baseline characteristics, including age, sex, body mass index, American Society of Anesthesiologists’ score, are shown in Table 1.
Table 1
| Patient characteristics | Total (n=210) |
|---|---|
| Sex (F:M) | 136:74 (65%:35%) |
| Age (years) | 48.3±15.4 |
| BMI (kg/m2) | 23.17k3.4 |
| ASA (I:II:III) | 33:169:8 |
| Abdominal pain | 51 (24.3%) |
| Benign:malignant | 120:90 |
| Charlson comorbidity index | 2.57±1.38 |
Data are number (% of group total) or mean (SD). RDP, robotic distal pancreatectomy; BMI, body-mass index; ASA, American Society of Anesthesiologists’ score; SD, standard deviation.
Pathological outcome
The mean largest tumor diameter was 2.5 cm, and 36 patients had a tumor larger than 5 cm (Table 2). The leading indication for RDP was a PDAC (n=73). The final histopathological examinations of the tumors were: PDAC (73, 34.8%), serous cystadenoma (29, 13.8%), mucinous cystadenoma (33, 15.7%), mucinous cystadenocarcinoma (8, 3.8%), solid-pseudopapillary tumor (32, 15.2%), neuroendocrine tumor (23, 11.0%), pancreatic pseudocyst (8, 3.8%) intraductal papillary mucinous neoplasm (4, 1.9%).
Table 2
| Pathological characteristics | Mean ± SD or No. (%) of patients |
|---|---|
| Mean largest tumor diameter (cm) | 2.5n la |
| Tumor ≥u cm | 36 (17.1) |
| Indications | |
| Ductal adenocarcinoma | 73 (34.8) |
| Mucinous cystadenoma | 33 (15.7) |
| Solid-pseudopapillary tumor | 32 (15.2) |
| Serous cystadenoma | 29 (13.8) |
| Neuroendocrine tumor | 23 (11.0) |
| Pseudocyst | 8 (3.8) |
| Mucinous cystadenocarcinoma | 8 (3.8) |
| IPMN | 4 (1.9) |
RDP, robotic distal pancreatectomy; SD, standard deviation; IPMN, intraductal papillary mucinous neoplasm.
Perioperative outcomes
Perioperative outcomes for RDP group are shown in Table 3. The mean operative time (includes docking and undocking of the robot) was 159.8 minutes, and the median EBL was 161.2 ± 262.2 mL. Nine patients required blood transfusion during the operations. When adenocarcinoma was excluded from analysis, the rates of spleen preservation and SVP were 80.7% (98/120) and 35.0% (42/120), retrospectively. Ten patients were converted to open operation because of excessive blood loss. In this study, postoperative morbidities occurred in 34 patients (16.2%). Among which, 12 patients had grade B pancreatic fistulas and 4 patients had grade C pancreatic fistulas. Two patients who experienced intra-abdominal bleeding required a second operation. The mean postoperative hospital stay was 8.5 days. No patients required readmission to hospital and there was no 30-day mortality.
Table 3
| Parameters | All (n=210) | Patients with benign tumors (n=120) |
|---|---|---|
| Operative characteristics | ||
| Operative time (min) | 159.8±65.5 | |
| EBL (mL) | 161.2±262.2 | |
| Transfusion | 9 (4.3) | |
| Conversion | 10 (4.8) | |
| SP | 131 (62.4) | 98 (80.7) |
| SVP | 57 (27.1) | 42 (35.0) |
| Postoperative outcomes | ||
| Morbidities | 34 (16.2) | |
| POPF | 30 (14.3) | |
| POPF (Grade B or C) | 16 (7.6) | |
| Clavien-Dindo classification III–V | 14 (6.7) | |
| Reoperation | 2 (1.0) | |
| Postoperative hospital stay (d) | 8.5±6.0 |
RDP, robotic distal pancreatectomy; EBL, estimated blood loss; SP, splenic preservation; SVP, splenic vessel preservation; POPF, postoperative pancreatic fistula.
Discussion
Laparoscopic distal pancreatectomy (LDP) is a commonly accepted approach to manage lesions in the body and tail of the pancreas (13-18). However, laparoscopic surgery has several technical limitations, such as reduced dexterity of manipulation and narrowed 2-dimensional visualization (6,19). Robotic surgery has recently been developed to overcome the aforementioned limitations of laparoscopic surgery by the flexible robotic arms and improved three-dimensional visualization (20,21). The robotic technique enabled precise dissection of the tumors which were located in deep, narrow spaces and allowed safe resection without injury to adjacent major vessels.
Previous studies have demonstrated that RDP can improve the clinical outcomes and reduce the risk of conversion to laparotomy when compared with LDP (4-6). However, the limitations of these studies include small sample sizes, long time span and inconsistent surgical techniques. Our series represents, to our knowledge, the largest single-institution report of clinical experience in RDP. This study demonstrates that RDP is safe and feasible with a low conversion rate, acceptable operative time, minimal blood loss, good postoperative outcomes and reduced postoperative hospital stay.
Docking the robot and exchanging instruments increased the time of robotic surgery (22). Pervious meta-analyses demonstrated that RDP was associated with longer mean operative times. Although 34.8% patients in this study had PDAC, our OT was also shorter when compared with other studies (4,6,16,23), which might by due to our high-volume institution having more experience in robotic techniques.
Previous studies have already demonstrated that RDP can significantly reduce the rate of conversion to open surgery when compared with LDP. In our study, 4.8% of patients who underwent RDPS required conversion to laparotomy, which was consistent with results reported in other studies (4-6). The lower conversion rate to open surgery in the robotic group might be related to the advantages in using the robotic techniques in vessel dissection and bleeding control.
We preferred spleen preservation and the Kimura technique for patients with non-malignant tumors. For spleen-preserving and splenic vessel preserving operations, delicate manipulation and good visualization is required as even a small break in the tributary vessels can necessitate a splenectomy or Warshaw procedure (24,25). Due to robotics can dissect vessels precisely and control excessive bleeding timely, our study demonstrated that RDP can reduce EBL and improve the rates of SP and SVP compared with the previous values in the literature (4,6).
In our study, robotic assistance did not decrease the postoperative hospital stay, the occurrence of pancreatic fistula and morbidity rates, but the severity of pancreatic fistula in RDP group was reduced when compared with earlier studies (26). All these might be related to that RDP do not require complex dissection and reconstruction, which have not taken full use of the robotic system.
This study has several limitations. First, this is a retrospective study. All data regarding patient demographics as well as perioperative outcomes were retrospectively collected from medical records. Second, this is a case series with its inherent defects. If the financial burden of robotic surgery is reduced, we may conduct a randomized controlled trial to investigate the benefits of RDP over LDP in the future.
Conclusions
In conclusion, this study demonstrated that RDP is safe and feasible, even when tumors are malignant. As the robotic surgical experience accumulated, the robotic system might be a useful approach for surgeons to perform RDP with spleen preservation, even SVP after training.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jin He) for the series “Robotic Surgery for Pancreatic Cancer” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2018.03.01). The series “Robotic Surgery for Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Institutional Review Board of the People’s Liberation Army General Hospital (S2016-098-01). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004;363:1049-57. [Crossref] [PubMed]
- Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol 2015;21:3157-65. [Crossref] [PubMed]
- Hanly EJ, Talamini MA. Robotic abdominal surgery. Am J Surg 2004;188:19S-26S. [Crossref] [PubMed]
- Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg 2013;257:128-32. [Crossref] [PubMed]
- Chen S, Zhan Q, Chen JZ, et al. Robotic approach improves spleen-preserving rate and shortens postoperative hospital stay of laparoscopic distal pancreatectomy: a matched cohort study. Surg Endosc 2015;29:3507-18. [Crossref] [PubMed]
- Kang CM, Kim DH, Lee WJ, et al. Conventional laparoscopic and robot-assisted spleen-preserving pancreatectomy: does da Vinci have clinical advantages? Surg Endosc 2011;25:2004-9. [Crossref] [PubMed]
- Venkat R, Edil BH, Schulick RD, et al. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg 2012;255:1048-59. [Crossref] [PubMed]
- Zhang YH, Zhang CW, Hu ZM, et al. Pancreatic cancer: Open or minimally invasive surgery? World J Gastroenterol 2016;22:7301. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [Crossref] [PubMed]
- Jean-Philippe A, Alexandre J, Christophe L, et al. Laparoscopic spleen-preserving distal pancreatectomy: splenic vessel preservation compared with the Warshaw technique. JAMA Surg 2013;148:246-52. [Crossref] [PubMed]
- Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg 2007;204:244-9. [Crossref] [PubMed]
- Jayaraman S, Gonen M, Brennan MF, et al. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg 2010;211:503-9. [Crossref] [PubMed]
- Jusoh AC, Ammori BJ. Laparoscopic versus open distal pancreatectomy: a systematic review of comparative studies. Surg Endosc 2012;26:904-13. [Crossref] [PubMed]
- Stauffer JA, Rosales-Velderrain A, Goldberg RF, et al. Comparison of open with laparoscopic distal pancreatectomy: a single institution's transition over a 7-year period. HPB (Oxford) 2013;15:149-55. [Crossref] [PubMed]
- Song KB, Kim SC, Park JB, et al. Single-center experience of laparoscopic left pancreatic resection in 359 consecutive patients: changing the surgical paradigm of left pancreatic resection. Surg Endosc 2011;25:3364-72. [Crossref] [PubMed]
- Kim SC, Park KT, Hwang JW, et al. Comparative analysis of clinical outcomes for laparoscopic distal pancreatic resection and open distal pancreatic resection at a single institution. Surg Endosc 2008;22:2261-8. [Crossref] [PubMed]
- Sanchez-Cabus S, Adam JP, Pittau G, et al. Laparoscopic left pancreatectomy: early results after 115 consecutive patients. Surg Endosc 2016;30:4480-8. [Crossref] [PubMed]
- Kooby DA, Hawkins WG, Schmidt CM, et al. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg 2010;210:779-85, 86-7.
- Garcia-Ruiz A, Gagner M, Miller JH, et al. Manual vs robotically assisted laparoscopic surgery in the performance of basic manipulation and suturing tasks. Arch Surg 1998;133:957-61. [Crossref] [PubMed]
- McDougall EM, Soble JJ, Wolf JS Jr, et al. Comparison of three-dimensional and two-dimensional laparoscopic video systems. J Endourol 1996;10:371-4. [Crossref] [PubMed]
- Boggi U, Signori S, De Lio N, et al. Feasibility of robotic pancreaticoduodenectomy. Br J Surg 2013;100:917-25. [Crossref] [PubMed]
- Stauffer JA, Coppola A, Mody K, et al. Laparoscopic Versus Open Distal Pancreatectomy for Pancreatic Adenocarcinoma. World J Surg 2016;40:1477-84. [Crossref] [PubMed]
- Hwang HK, Kang CM, Chung YE, et al. Robot-assisted spleen-preserving distal pancreatectomy: a single surgeon's experiences and proposal of clinical application. Surg Endosc 2013;27:774-81. [Crossref] [PubMed]
- Shoup M, Brennan MF, McWhite K, et al. The value of splenic preservation with distal pancreatectomy. Arch Surg 2002;137:164-8. [Crossref] [PubMed]
- Gavriilidis P, Lim C, Menahem B, et al. Robotic versus laparoscopic distal pancreatectomy - The first meta-analysis. HPB (Oxford) 2016;18:567-74. [Crossref] [PubMed]
Cite this article as: Liu Q, Tang W, Zhou R, Zhao Z, Gao Y, Hu M, Li C, Zhang X, Liu R. Robotic distal pancreatectomy: experience in a high-volume center. Ann Pancreat Cancer 2018;1:15.