
Technical aspects of modern radiation therapy for pancreatic adenocarcinoma: field design, motion management, dosing, and concurrent therapy
Borderline resectable or locally advanced disease
Rationale
Roughly 30–40% of patients with newly diagnosed pancreatic adenocarcinoma present with either locally advanced or borderline resectable disease (1). While borderline resectable tumors can be technically resected, the presence of vessel involvement confers both a higher risk of positive margins with upfront resection as well as a higher risk of distant relapse presumably due to micrometastatic disease (2-6). As such, neoadjuvant systemic therapy is recommended to help sterilize the tumor-vessel interface and address micrometastatic disease. Although the optimal duration of neoadjuvant systemic therapy has yet to be determined for patients with borderline disease, there is data to suggest that patients who receive greater than 4 months of multi-agent chemotherapy experience improved outcomes (7). Following systemic therapy, radiation can be considered to further enhance the likelihood of margin negative resection and decrease local recurrence, which is supported by single-arm studies (8-14). This approach is currently being studied in the phase III setting (15).
Patients with locally advanced disease have traditionally been considered unresectable. The advent of multi-agent chemotherapy and improved radiation techniques have given surgical teams more confidence in considering exploration for patients with locally advanced disease (16). Recent institutional series from high-volume centers have reported much higher rates of margin negative resections in patients with locally advanced disease as compared to what has been historically reported in randomized trials, lending credence to the concept of “neoadjuvant” therapy for patients with locally advanced disease (17,18). Outside of this paradigm, the role of radiation therapy is highly controversial, but can be administered as consolidation therapy following completion of systemic therapy to prevent local progression and palliate pain (19). While the impact of radiation in this setting on systemic spread and overall survival is less clear, the potential importance of local control was highlighted by the finding that mortality from pancreatic cancer may be driven by complications related to the primary tumor in up to 30% of patients (20).
In both the borderline resectable and locally advanced setting, chemoradiation with standard fractionation over 5 to 6 weeks has been the historical approach. More recently, hypofractionated stereotactic body radiation therapy (SBRT) and dose-escalated intensity modulated radiation therapy (IMRT) have gained favor. Herein, we describe technical considerations relevant for use of these radiation modalities.
Conventional chemoradiation
Patients undergoing conventional chemoradiation are simulated supine using a thin slice (≤3 mm) computed tomography protocol. Immobilization can consist of an Alpha Cradle (Smithers Medical Products Inc., North Canton, OH, USA) with wing-board (CIVCO Medical Solutions, Coralville, IA, USA) or equivalent device that allows reproducible immobilization of the patient with arms out of the radiation field. Patients should be asked to fast 3 to 5 hours prior to simulation, as well as prior to each fraction, to allow reproducible gastric positioning, as the stomach is a highly distensible organ which can impact pancreatic motion (21). Indeed, the pancreas has been shown to move significantly when the stomach is injected with 500 to 900 mL of liquid, with the pancreatic tail and SMA showing the greatest motion (22). For better visualization of GI luminal structures, oral contrast can be administered, but the same amount of volume of water should also be administered prior to each fraction to maintain reproducibility. Intravenous contrast should also be administered to aid in target delineation in the absence of contraindications. Special attention should be given to selection of the optimal contrast agent, contrast dosing, injection rate, and CT sim scanner settings (i.e., kVp and mAs) (23). Scan acquisition timing with respect to intravenous contrast injection should be optimized based on the clinical scenario, with late arterial, pancreatic, and portal venous phases occurring roughly 35, 45, and 70 seconds after injection (24).
Multiple studies demonstrate significant pancreatic motion during the respiratory cycle, particularly in the superior-inferior (SI) and anterior-posterior (AP) directions. Pancreatic motion in the SI direction has been measured at 0.5–2.4 cm, although instances of >4.0 cm have been reported, while excursion in the AP and left-right (LR) directions have been reported at 0.5–2.4 and 0.07–0.6 cm, respectively (25-32). Variation in motion between the head, body, and tail of the pancreas has been illustrated, as has variation in motion of the local vasculature (33-35). Clearly, respiratory motion is a major source of intrafractional variability for pancreatic tumors, highlighting the need for individualized margins and/or motion management strategies. Several motion management strategies exist, including respiratory gating, breath-hold [active breathing control (ABC)], respiratory tracking, or abdominal compression (discussed in more detail below). Given the number of fractions for conventional chemoradiation, a four-dimensional (4D)-CT is often used to determine a patient-specific internal tumor volume (ITV) rather than employing motion management strategies. Notably, 4D-CT may both underestimate and overestimate true respiratory motion, as compared to fluoroscopy, ultrasound, or cine-MRI, with dependency on technique and patient comfort (31,36).
Elective nodal irradiation (ENI) is controversial for conventional chemoradiation and has fallen out of favor. Generally, the primary tumor and clinically positive lymph nodes (>1 cm) should be contoured as the gross tumor volume, with assistance from both anatomical and functional imaging. Maximum and minimum excursions can be defined from the 4D-CT and fused with the primary CT to allow construction of an ITV, acknowledging the limitations of 4D-CT (35). Use of a clinical tumor volume (CTV) to account for microscopic extent of disease is controversial, as are appropriate expansions when a CTV is constructed. Commonly, margins of 0.5–1.5 cm are used when creating a CTV. Planning tumor volume (PTV) margins are similarly variable and institution-specific and depend on immobilization, motion management, and method of image guidance. With respect to image guidance, cone beam CT (CBCT) can be used for bone and fiducial setup, although soft tissue structures, including tumor, are usually not easily visible on CBCT. If an ITV has been constructed and daily image guidance with CBCT is utilized, PTV margins can be reduced to 5–7 mm, whereas margins up to 2 cm should be considered if such tools are not used.
Prescription dose is most commonly 50.4–54 Gy administered in 1.8–2.0 Gy fractions over 5.5–6 weeks, primarily limited by dose to the stomach and small bowel. Patients enrolled on the most recent LAP07 trial received 54 Gy in 30 fractions (19). Critical organs at risk (OARs) that should be contoured include stomach, bowel, liver, kidneys, and spinal canal/cord. The original Emami estimate of TD5/5 and TD50/5 for whole organ irradiation to the stomach were 50 and 65 Gy, respectively, while these values for irradiation to a partial small bowel volume were 50 and 60 Gy, respectively (37). More recently, the stomach and small bowel section of the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) acknowledged the paucity of high-quality clinical data on the subject, noting insufficient data to make firm dose-constraint recommendations (38). The QUANTEC report also pointed out that the manner in which the bowel is contoured, either as individual loops or as a “bowel bag”, will influence the relevant constraints. Volumetric constraints were suggested for both methods of contouring, namely V45<195cc when a bowel bag is contoured and V15<120cc when individual loops of bowel are contoured. However, it should be noted that these volumetric recommendations were based on acute toxicity endpoints from single studies with limited number of patients who were undergoing either whole pelvic radiation for gynecologic malignancies or pre-operative radiation for rectal cancer, rendering their applicability to patients undergoing chemoradiation for pancreatic cancer questionable (39,40). A more recent study from MD Anderson specifically examined late duodenal toxicity in patients undergoing chemoradiation for unresectable pancreatic cancer and suggested that the duodenal V55<1cc was an important independent dosimetric predictor, with a maximum dose of 60 Gy also potentially driving toxicity (41). Ultimately, more toxicity data is required to better refine recommendations. In the meantime, the constraints listed in Table 1 are reasonable to follow.
Table 1
| Organ at risk | Constraints |
|---|---|
| Liver | Mean dose <25 Gy, V20<30 |
| Kidney | Mean <18 Gy, two-thirds each kidney <20 Gy |
| Stomach | Dmax <55 Gy (can likely tolerate higher point doses) |
| Bowel | V55<1cc, V45<15 |
| Bowel loops: V45<195cc | |
| Individual bowel: V15<120cc | |
| Cord | Dmax <45 Gy |
Both three-dimensional conformal radiation therapy (3D-CRT) and IMRT are acceptable planning techniques, but IMRT is becoming the standard based on data showing more conformal target coverage, decreased dose to OARs, and decreased toxicity (42-44). IMRT may also allow dose escalation, as illustrated by a phase I/II trial in which dose escalation to 55 Gy in 25 fractions with concurrent gemcitabine was feasible (45). More recent exploration into much higher dose escalation is described in more detail below.
Multiple radiosensitizing agents have been evaluated concurrently with radiation, most commonly gemcitabine and 5-fluorouracil (5-FU). Retrospective data from MD Anderson suggested increased acute toxicity with gemcitabine as compared to 5-FU (46). More recently, the phase II SCALOP trial compared gemcitabine-based chemoradiation with capecitabine-based chemoradiation, and showed improved outcomes and decreased toxicity with the capecitabine-based regimen (47). Although the results of the SCALOP trial should be interpreted cautiously given issues related to inconsistent treatment administration, the excellent tolerability of capecitabine, along with its ease of administration, has made it the most commonly used concurrent agent.
SBRT
SBRT offers several advantages over conventional chemoradiation, including delivery over 3–5 days as compared to 5–6 weeks, preservation of local control with minimal interruption of chemotherapy and minimal delay until surgery, and decreased acute toxicity (48). The focused radiation field may also induce less fibrotic changes as compared to conventional chemoradiation, which is important when considering subsequent surgical exploration. Furthermore, SBRT may offer the opportunity for dose escalation, particularly along the tumor-vessel interface, which may increase the likelihood of margin negative resections and local control.
Optimal dose and fractionation for pancreatic SBRT has not been determined, nor have dose tolerances for relevant dose-limiting structures, namely, stomach, duodenum, and bowel. Initial studies with SBRT explored single-fraction treatment using a 25 Gy × 1 regimen. While this resulted in excellent local control with rates of freedom from local progression >90%, high rates of late grade 2–4 GI toxicity were seen (49-53). Analysis of potential dosimetric predictors of late grade 2 or higher duodenal toxicity for single-fraction treatment suggested V15>9cc, V20>3cc, and maximum dose >23 Gy as important thresholds associated with increased toxicity (54).
Given the high rate of toxicity seen with single fraction treatment, a multi-institutional trial was designed which employed a hypofractionated SBRT approach of 6.6 Gy × 5 with the goal of preserving local control while reducing late toxicity to an acceptable rate. With the fractionated approach, normal tissue BED3 was decreased from 233.3 Gy with the single fraction approach to 105.6 Gy with the five-fraction approach. Specific dose constraints were based on the single fraction experience, employing the V15 and V20 referenced above as well as V33<1cc. Acceptable rates of acute (2%) and late (2%) GI toxicity were seen, while global quality-of-life (QOL) was preserved and pain scores were improved (55). Despite a decrease in BED10 from 87.5 to 54.8 Gy, freedom from local progression at 1 year was still 78%. Mahadevan et al. also reported reasonable outcomes and toxicity using a three-fraction regimen, in which the daily fractional dose was based on the proximity of the target to bowel, ranging from 8 Gy per fraction for those patients with significant contact between tumor and duodenum to 12 Gy per fraction for those patients with >3 mm of separation (56). In this study, a 30-Gy dose constraint was used for the stomach and bowel, which is derived from the liver SBRT experience and was also endorsed by in the QUANTEC report (38,57,58).
Simultaneous integrated boost (SIB) techniques have been used to further escalate the dose to portions of tumor away from the stomach and bowel, particularly at tumor-vessel interfaces, which may help achieve subsequent margin negative resection. Using this technique, investigators at Moffitt were able to escalate dose to the tumor and tumor-vessel interface from 25 and 35 Gy, respectively, to 30 and 40 Gy, respectively, while maintaining late grade 3 or higher radiation-related toxicity to <10% (9,10). More recently, investigators at Emory conducted a phase I trial in which dose to the tumor and posterior margin was escalated to 36 and 45 Gy, respectively, in three fractions with acceptable toxicity, although it should be noted that only tumors that were >3 mm away from stomach and bowel were included in this trial (59). Notably, the dose constraints used in these trials were different than the aforementioned studies, highlighting the fact that more robust data on optimal dose constraints for pancreas SBRT are needed. For example, it is likely that the five fraction constraints for stomach and bowel utilized in the multi-institutional trial referenced above can be liberalized, as is being done in the ongoing Alliance trial for borderline resectable patients, in which V35<1cc and V20<20cc are being used without limitation on the V15 (15). Use of proton or carbon ion therapy may allow further dose escalation but require further validation (60-63).
Many of the technical considerations referenced above with respect to the delivery of conventional chemoradiation also apply to SBRT but take on greater importance given the potential for injury with higher fractional dose. Given limited soft tissue resolution with fluoroscopy and cone beam CT, fiducial placement prior to simulation is critical to track tumor motion given high interfraction and intrafraction variability form respiratory motion and bowel gas patterns. Although both percutaneous and endoscopic placement are viable options, endoscopic administration under ultrasound guidance is preferred. Fiducials placed in this manner have been shown to generally remain stable throughout treatment (64). Several fiducial vendors exist, and more data is needed that compares the stability, visualization, and artifact between fiducial types. While biliary stents may appear to serve as a surrogate for pancreatic motion, stent migration and spontaneous loss are possible, and stent motion may be greater than gross tumor volume (GTV) motion, rendering stents less reliable surrogates for tumor tracking as compared to fiducials (28,64).
Simulation should proceed as noted above, but thin slice CT (≤2 mm) should be acquired for SBRT cases. While most pancreatic tumors will show significant excursions with respiration as motioned above, 4D-CT can be used at time of simulation to quantify respiratory motion. If tumors show greater than 3 mm of motion with respiration on 4D-CT, motion management techniques should be employed. Motion management can include immobilization of the target, through breath-hold techniques or abdominal compression, or tumor monitoring, through tracking or gating. Regarding the former approach, abdominal compression limits diaphragmatic motion, but should be used with caution as it can occasionally compress stomach or bowel into the pancreas, increasing dose to OARs. Both inspiration and expiration breath-hold techniques can be used, but expiration breath-hold may offer dosimetric benefits with respect to OAR dose (65). If breath-hold technique is utilized, multiple CT scans in breath-hold can be acquired at time of simulation to assess the reproducibility of breath-hold and inform margins. For the rare patients with <3 mm of excursion on 4D-CT, the average CT, maximum/minimum intensity projections (MIPs), or fusion of the minimum and maximum excursion phases can be used to generate an ITV.
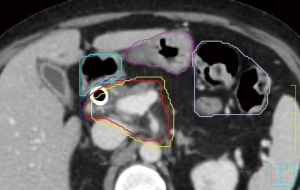
Variation across institutions exists with respect to target volume delineation. At our institution, the gross tumor volume is contoured on the treatment planning CT after review of available diagnostic anatomic and functional imaging (66). In the absence of motion management techniques, the GTV is expanded to create an ITV based on the 4D-CT. If motion management techniques are employed, the GTV is expanded directly to the PTV. PTV margins are institution-specific, but should be on the order of 3–5 mm. Stomach, duodenum, and bowel are contoured on all axial slices of the PTV contour as well as one centimeter below and above the PTV. These GI structures are subsequently expanded by 2 mm, and this expanded volume is subtracted from the PTV to create a “modified PTV”. Coverage goals are established for the GTV, ITV, PTV, and modified PTV based on proximity to OARs. Figure 1 shows an example of this contouring approach. Other OARs that should be contoured include the kidneys, liver, and spinal canal/cord. Additionally, fiducials and clips should be contoured with appropriate ITV and PTV margins.
Linac-based treatment can be administered using multiple coplanar isocentric beams, but non-coplanar non-isocentric treatment can also be considered (51). Generally, high-energy photons are preferred as lower energy beams may result in more gastrointestinal toxicity. Isocentric treatment can be delivered using step and shoot IMRT or volumetric modulated arc therapy (VMAT), with the latter offering the benefit of decreased treatment time, an important variable to consider when employing motion management strategies such as breath-hold which may be taxing on the patient. Similarly, use of flattening filter free (FFF) beams may also decrease total treatment time (67).
As noted above, appropriate image guidance is critical for tumor tracking and patient positioning. Cone beam CT can be used to first align to spine to allow for rotational corrections. Robotic couches that allow for automated rotational corrections may be helpful in this process. After spinal alignment, translational shifts can be subsequently made to align the fiducials. Alternately, direct shifts to the fiducial can be made after laser alignment, but one should still be mindful of the degree of rotational inaccuracy. Whether translational adjustments can make up for rotational offsets, as has been show for tumors of the liver, should be further explored (68).
More recently, investigators have reported their experience using MRI-guided linear accelerators to administer SBRT for pancreatic cancer (69-73). MRI-based image guidance offers far superior soft tissue delineation as compared to traditional cone beam CT. Additionally, MRI-guided linear accelerators can offer the opportunity for adaptive replanning if significant interfraction variation is seen in tumor or OAR positioning. Indeed, the early experience with use of MRI-guided linear accelerators for pancreatic cancer has confirmed that significant interfraction variation in GI anatomy exists, which a significant proportion of patients requiring adaptive replanning. In these patients, treatment with the initial beam orientation would have resulted in significantly increased dose to OARs and decreased tumor coverage, highlighting the potential for MRI-based linear accelerators with adaptive replanning to offer safer and more effective treatment. Longer follow-up will be required to determine if the improvements in dosimetry that are obtained with MRI-based adaptive replanning translate into improved clinical outcomes (74).
Dose-escalation through fractionation
As noted above, SBRT allows precise delivery of high doses of radiation to small tumor volumes. SBRT is ideally suited for tumors in parallel organs such as the lung or the liver since ablation of small portions of these organs do not result in overall impairment in organ function. The pancreas, however, is surrounded by serially functioning OARs, namely the stomach and bowel, which limit delivery of truly ablative doses of radiation. Whereas deliver of BED10 values of >100 Gy are considered ablative across other tumor types, current SBRT dose prescriptions, for example 33 Gy in 5 fractions, deliver roughly half of this value. As such, while SBRT using current dose prescriptions may offer a convenient manner in which to deliver BED values similar to conventional chemoradiation, its impact on oncologic outcomes may be more limited. Indeed, while comparisons of SBRT to conventional chemoradiation have shown improvements in toxicity due to the focused nature of the SBRT field, significant improvements in oncologic endpoints have not been reported to date (48).
Investigators at MD Anderson have combined the principle of fractionation with the SBRT technique, inhomogeneous target coverage, and adaptive planning to deliver more ablative doses of radiation to pancreatic tumors (75,76). Fractionation allows repair of sublethal normal tissue damage in between treatments, increasing the total dose that can be administered. Fractionation also renders the treatment more resistant to daily variation in organ motion and tumor position. Even with fractionation, however, it is not possible to administer ablative doses to the entire tumor, as portions of pancreatic tumors are almost always abutting or in close proximity to GI luminal structures. A novel concept, therefore, is whether inhomogeneous treatment of part of the tumor with ablative doses using a SIB technique can improve oncologic outcomes. In this approach, the periphery of the tumor that abuts or is in close proximity to GI luminal structures receives a conventional dose, while the tumor center receives an ablative dose.
Under the MD Anderson approach, the number of fractions administered is based on the minimum distance of the tumor from the nearest GI luminal structure. If this distance is less than 1 cm, treatment is administered over 25 fractions, whereas if this distance is greater than 1 cm, 15 fractions are used. The GTV and areas of potential microscopic extension around the tumor, celiac axis, and superior mesenteric artery are treated to 45 Gy in 25 fractions or 37.5 Gy in 15 fractions, using a 10 mm CTV expansion and a 5 mm PTV expansion. A SIB volume is subsequently created by expanding the GTV 0–5 mm to create an initial PTV, with the exact margin determined by proximity to bowel, and subsequently subtracting out a planning structure that is created by applying a 5 mm expansion on the 4D contour of proximal GI structures. This SIB volume is treated either to 75 Gy in 25 fractions or 67.5 Gy in 15 fractions. An additional SIB volume is occasionally created through a further 5–10 mm contraction of the initial boost volume and treated to 100 Gy in 25 fractions or 75 Gy in 15 fractions.
Given the ablative doses used with this approach, careful attention to tumor and organ motion is critical through use of fiducials and reliable motion management techniques, as described above. The investigators at MD Anderson have also had access to CT on rails which allows greater soft tissue delineation and day-to-day assessment of OAR positioning, with consideration of adaptive replanning if significant variation in anatomy exists as compared to the simulation CT. In the future, MRI-based image guidance may further assist in this process. While longer follow-up with this approach is needed, early reports are encouraging and highlight the importance of radiation dose for local control, with a potential impact on overall survival, particularly in the era of better systemic control with multi-agent chemotherapy (77).
Adjuvant chemoradiation
Rationale
Although benefit to adjuvant chemotherapy has been demonstrated, the role of adjuvant chemoradiation beyond adjuvant chemotherapy for resected pancreatic cancer is less clear. Multiple historical trials have examined this question with conflicting results, although their applicability to the modern management of pancreatic cancer patients is uncertain given the older radiation techniques that were used in these studies (78-84). The importance of radiation quality was demonstrated in RTOG 9704, in which patients who were treated with radiation that violated protocol guidelines experienced inferior overall survival (85). Perhaps more importantly, while distant failure predominates after resection for pancreatic cancer, multiple studies over time have consistently shown that local recurrence, either in isolation or concurrent with distant recurrence, significantly contributes to overall patterns of failure (86-89). In fact, in the recently published ESPAC-4 trial, which randomized resected patients to either adjuvant gemcitabine and xeloda or adjuvant gemcitabine monotherapy, roughly 50% of patients in both arms experienced local recurrence, with over half of these local recurrences being isolated in nature (90). Moreover, roughly 60% of patients enrolled in ESPAC-4 underwent R1 resections, which highlights the fact that R1 resection rates continue to be high in patients with localized pancreatic cancer. These findings support consideration of adjuvant chemoradiation after completing adjuvant chemotherapy, particularly in patients with risk factors for local recurrence, such as close or positive margins and/or positive lymph nodes, and who maintain a good performance status after completing adjuvant chemotherapy. Ultimately, the ongoing RTOG 0848 will help clarify the value of adjuvant chemoradiation beyond adjuvant chemotherapy alone (91).
Concurrent chemotherapy
Historically, adjuvant radiation has been most commonly administered with 5-FU, although other concurrent agents have been explored. Several studies explored the combination of 5-FU, cisplatin, and interferon alpha with promising results (92-94). Moreover, after gemcitabine was proven successful in the adjuvant setting, exploration of gemcitabine-based chemoradiation was also studied and shown to be feasible, also with promising results as compared to historical controls (95). Indeed, modern conformal radiation with IMRT has allowed intensification of concurrent chemotherapy and consideration of new concurrent agents. As an example, administration of erlotinib concurrently with capecitabine-based chemoradiation was shown to be feasible, although erlotinib has now fallen out of favor given negative results in LAP-07 (96). More recently, there is been interest in incorporating immunotherapeutic agents into the adjuvant setting, including anti-CD40 agents, vaccines targeting tumor-specific Ras mutations, granulocyte-macrophage colony-stimulating factor (GM-CSF) vaccines, or agents targeting telomerase (97-99). Whether radiation can further enhance immunotherapeutic effects is unclear and under investigation (100). Currently, NCCN guidelines offer 5-FU (continuous infusion or oral capecitabine) and gemcitabine as options for concurrent chemotherapy with radiation, although the ease of capecitabine administration and its excellent tolerability make it the preferred choice.
Technical delivery of adjuvant radiation
CT simulation should proceed in a similar fashion as described above for conventional chemoradiation for locally advanced or borderline resectable disease. Patients are simulated supine using a thin slice (≤3 mm) CT protocol in alpha cradle/wingboard or similar immobilization device with arms out of the field. IV contrast should be administered unless contraindication. Oral contrast can be used to visualize GI luminal structures, with the same amount of water given to patients prior to treatment. Patients should be asked to fast 3 to 5 hours prior to simulation, as well as each fraction, to allow reproducible gastric positioning. Motion management considerations are as noted above and most commonly addressed by using a 4D-CT with fusion of the maximum and minimum excursion phases.
Prior to initiating contouring, it is imperative to review pre-operative and post-operative diagnostic imaging as well as operative and pathology documentation. In the absence of gross recurrent disease, there is no GTV contour. A consensus contouring atlas from the RTOG is available for CTV delineation, which is consistent with the RTOG 0848 protocol (101). An upper abdominal normal organ consensus contouring atlas is also available (102). The CTV includes the celiac, sma, porta hepatis, and para-aortic lymph node chains, as well as the tumor bed, and relevant anastomoses. Usually, the Pancreaticojejunostomy (PJ) is the only anastomosis that needs to be included in the field, but in the instance of close or positive bile duct margins, the hepatico-jejunostomy (HJ) will also need to be included. The PJ can be identified by following the pancreatic remnant, while the HJ can be identified by following air in the intrahepatic biliary tree down to the hilum. A summary of the steps to create the CTV, along with relevant expansions, is included in Table 2. For patients with body or tail lesions who have undergone a distal pancreatectomy or splenectomy, the data for adjuvant radiation is sparse, although some evidence supporting adjuvant radiation in patients with risk factors for recurrence, most notably lymph node positive disease (103). For these patients, the splenic/hilar nodes should be included in the CTV and the porta hepatis nodes can likely be withheld.
Table 2
| Target volume | Instructions |
|---|---|
| CTV | Components: |
| (I) Post-operative bed (use pre-operative scan to help delineate); | |
| (II) Pancreaticojejunostomy, with 1 cm expansion; | |
| (III) Proximal 1–1.5 cm of celiac artery to bifurcation, with 1 cm expansion; | |
| (IV) Proximal 2.5–3.0 cm of SMA, with 1 cm expansion; | |
| (V) PV from first SMV/splenic vein confluence to first slice in which center of PV is to the right of the IVC, with 1cm expansion; | |
| (VI) Aorta from upper most PV, CA, or SMA to bottom of L2, or to bottom of L3 for low-lying tumors, with asymmetric expansions to cover pre-vertebral space (start with 2.5 cm to the right, 1 cm to the left, 0.2 cm posteriorly, 2 cm anteriorly). | |
| PTV | 5–7 mm expansion on CTV |
| PTV boost | To consider boosting area of close or positive margins to ≥54 Gy |
CTV, clinical tumor volume; PTV, planning tumor volume; SMA, superior mesenteric artery; PV, portal vein; SMV, superior mesenteric vein; CA, celiac artery.
Generally, a dose of 50.4 Gy is administered in 28 fractions, although a further boost, either as a cone-down or SIB, to 54 Gy or higher can be considered for close or positive margins, assuming that dose constraints can be maintained. As noted above, IMRT has been shown to decrease dose to OARS and result in decreased acute toxicity (42). Some investigators have also explored the use of SBRT in the adjuvant setting, which may be particularly useful for patients with close or positive margins, although more data is needed (100,104). Patients with a positive uncinate margin may particularly good candidates for adjuvant SBRT in the future, since the PJ and HJ would have to be targeted for positive pancreatic neck and bile duct margins respectively and may not be able to tolerate hypofractionated treatment as compared to the superior mesenteric vasculature. Recent data suggesting that the highest risk of local recurrence following pancreaticoduodenectomy is in close proximity to the celiac and SMA may allow further refinement and narrowing of adjuvant radiation fields, which may lend itself to more hypofractionated approaches in the future (89).
Conclusions
While the role of radiation for pancreatic cancer has historically been controversial, much of this controversy is based on older studies that employed antiquated radiation techniques and that are therefore less applicable to the question of the utility of modern radiation. Advances in motion management, image guidance, and treatment planning, as described above, have refined the delivery of radiation, lending optimism about the future role of radiation across all stages of non-metastatic pancreatic cancer, including adjuvant treatment for resected disease, neoadjuvant treatment for borderline resectable and more recently a fraction of locally advanced disease, and definitive therapy for unresectable disease. An understanding of the technical challenges that face radiation therapy for pancreatic cancer, along with current and potential solutions, is critical for the optimal integration of radiation into the multi-disciplinary management of patients with pancreatic cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Tuli) for the series “Radiotherapy and Pancreatic Adenocarcinoma” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2018.07.06). The series “Radiotherapy and Pancreatic Adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mauro LA, Herman JM, Jaffee EM, et al. Carcinoma of the Pancreas. Abeloff's Clinical Oncology Review. 5th ed. Philadelphia, PA: Elsevier Inc., 2013:1397.
- Katz MH, Marsh R, Herman JM, et al. Borderline resectable pancreatic cancer: need for standardization and methods for optimal clinical trial design. Ann Surg Oncol 2013;20:2787-95. [Crossref] [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46. [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- Yamada S, Fujii T, Sugimoto H, et al. Aggressive surgery for borderline resectable pancreatic cancer: evaluation of National Comprehensive Cancer Network guidelines. Pancreas 2013;42:1004-10. [Crossref] [PubMed]
- Rosati L, Hacker-Prietz A, Rao A, et al. Multiagent Induction Chemotherapy With Duration Longer Than 4 Months Improves Survival in Patients With Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma Who Received Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;96:S205-6. [Crossref]
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: alliance for clinical trials in oncology trial A021101. JAMA surgery 2016;151:e161137 [Crossref] [PubMed]
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516-22. [Crossref] [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [Crossref] [PubMed]
- Dholakia AS, Hacker-Prietz A, Wild AT, et al. Resection of borderline resectable pancreatic cancer after neoadjuvant chemoradiation does not depend on improved radiographic appearance of tumor–vessel relationships. J Radiat Oncol 2013;2:413-25. [Crossref] [PubMed]
- Moningi S, Dholakia A, Raman S, et al. Surgical Outcomes Following Chemotherapy and Stereotactic Body Radiation Therapy in Patients With Borderline and Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2014;90:S366-7. [Crossref]
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619-27. [Crossref] [PubMed]
- Lee JL, Kim SC, Kim JH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 2012;152:851-62. [Crossref] [PubMed]
- Katz MH, Ou F, Herman JM, et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer 2017;17:505. [Crossref] [PubMed]
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801-10. [Crossref] [PubMed]
- Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 2013;18:543-8. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem J, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Mahmood U, Errens M, Zimrin A, et al. The remarkably distensible stomach: Case report highlighting the implications of gastric filling on radiation treatment planning for gastric lymphoma. Pract Radiat Oncol 2012;2:265-9. [Crossref] [PubMed]
- Horst E, Micke O, Moustakis C, et al. Conformal therapy for pancreatic cancer: variation of organ position due to gastrointestinal distention—implications for treatment planning. Radiology 2002;222:681-6. [Crossref] [PubMed]
- Bae KT. Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 2010;256:32-61. [Crossref] [PubMed]
- Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014;270:248-60. [Crossref] [PubMed]
- Hallman JL, Mori S, Sharp GC, et al. A four-dimensional computed tomography analysis of multiorgan abdominal motion. Int J Radiat Oncol Biol Phys 2012;83:435-41. [Crossref] [PubMed]
- Shiinoki T, Shibuya K, Nakamura M, et al. Interfractional reproducibility in pancreatic position based on four-dimensional computed tomography. Int J Radiat Oncol Biol Phys 2011;80:1567-72. [Crossref] [PubMed]
- Cattaneo GM, Passoni P, Sangalli G, et al. Internal target volume defined by contrast-enhanced 4D-CT scan in unresectable pancreatic tumour: evaluation and reproducibility. Radiother Oncol 2010;97:525-9. [Crossref] [PubMed]
- Goldstein SD, Ford EC, Duhon M, et al. Use of respiratory-correlated four-dimensional computed tomography to determine acceptable treatment margins for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2010;76:597-602. [Crossref] [PubMed]
- Gwynne S, Wills L, Joseph G, et al. Respiratory movement of upper abdominal organs and its effect on radiotherapy planning in pancreatic cancer. Clin Oncol (R Coll Radiol) 2009;21:713-9. [Crossref] [PubMed]
- Minn AY, Schellenberg D, Maxim P, et al. Pancreatic tumor motion on a single planning 4D-CT does not correlate with intrafraction tumor motion during treatment. Am J Clin Oncol 2009;32:364-8. [Crossref] [PubMed]
- Feng M, Balter JM, Normolle D, et al. Characterization of pancreatic tumor motion using cine MRI: surrogates for tumor position should be used with caution. Int J Radiat Oncol Biol Phys 2009;74:884-91. [Crossref] [PubMed]
- Reese AS, Lu W, Regine WF. Utilization of intensity-modulated radiation therapy and image-guided radiation therapy in pancreatic cancer: is it beneficial? Semin Radiat Oncol 2014;24:132-9. [Crossref] [PubMed]
- Bhasin DK, Rana SS, Jahagirdar S, et al. Does the pancreas move with respiration? J Gastroenterol Hepatol 2006;21:1424-7. [PubMed]
- Li XA, Qi XS, Pitterle M, et al. Interfractional variations in patient setup and anatomic change assessed by daily computed tomography. Int J Radiat Oncol Biol Phys 2007;68:581-91. [Crossref] [PubMed]
- Mori S, Hara R, Yanagi T, et al. Four-dimensional measurement of intrafractional respiratory motion of pancreatic tumors using a 256 multi-slice CT scanner. Radiother Oncol 2009;92:231-7. [Crossref] [PubMed]
- Ge J, Santanam L, Noel C, et al. Planning 4-dimensional computed tomography (4DCT) cannot adequately represent daily intrafractional motion of abdominal tumors. Int J Radiat Oncol Biol Phys 2013;85:999-1005. [Crossref] [PubMed]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [Crossref] [PubMed]
- Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 2010;76:S101-7. [Crossref] [PubMed]
- Roeske JC, Bonta D, Mell LK, et al. A dosimetric analysis of acute gastrointestinal toxicity in women receiving intensity-modulated whole-pelvic radiation therapy. Radiother Oncol 2003;69:201-7. [Crossref] [PubMed]
- Baglan KL, Frazier RC, Yan D, et al. The dose-volume realationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2002;52:176-83. [Crossref] [PubMed]
- Kelly P, Das P, Pinnix CC, et al. Duodenal toxicity after fractionated chemoradiation for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2013;85:e143-9. [Crossref] [PubMed]
- Yovino S, Poppe M, Jabbour S, et al. Intensity-modulated radiation therapy significantly improves acute gastrointestinal toxicity in pancreatic and ampullary cancers. Int J Radiat Oncol Biol Phys 2011;79:158-62. [Crossref] [PubMed]
- Landry JC, Yang GY, Ting JY, et al. Treatment of pancreatic cancer tumors with intensity-modulated radiation therapy (IMRT) using the volume at risk approach (VARA): employing dose-volume histogram (DVH) and normal tissue complication probability (NTCP) to evaluate small bowel toxicity. Med Dosim 2002;27:121-9. [Crossref] [PubMed]
- Milano MT, Chmura SJ, Garofalo MC, et al. Intensity-modulated radiotherapy in treatment of pancreatic and bile duct malignancies: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2004;59:445-53. [Crossref] [PubMed]
- Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;84:1166-71. [Crossref] [PubMed]
- Crane CH, Abbruzzese JL, Evans DB, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advanced pancreatic cancer? Int J Radiat Oncol Biol Phys 2002;52:1293-302. [Crossref] [PubMed]
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317-26. [Crossref] [PubMed]
- Park JJ, Hajj C, Reyngold M, et al. Stereotactic body radiation vs. intensity-modulated radiation for unresectable pancreatic cancer. Acta Oncol 2017;56:1746-53. [Crossref] [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [Crossref] [PubMed]
- Koong AC, Christofferson E, Le Q, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2005;63:320-3. [Crossref] [PubMed]
- Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008;72:678-86. [Crossref] [PubMed]
- Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115:665-72. [Crossref] [PubMed]
- Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:181-8. [Crossref] [PubMed]
- Murphy JD, Christman-Skieller C, Kim J, et al. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys 2010;78:1420-6. [Crossref] [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. [Crossref] [PubMed]
- Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2010;78:735-42. [Crossref] [PubMed]
- Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol 2006;45:848-55. [Crossref] [PubMed]
- Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005;62:1371-8. [Crossref] [PubMed]
- Shaib WL, Hawk N, Cassidy RJ, et al. A phase 1 study of stereotactic body radiation therapy dose escalation for borderline resectable pancreatic cancer after modified FOLFIRINOX (NCT01446458). Int J Radiat Oncol Biol Phys 2016;96:296-303. [Crossref] [PubMed]
- Terashima K, Demizu Y, Hashimoto N, et al. A phase I/II study of gemcitabine-concurrent proton radiotherapy for locally advanced pancreatic cancer without distant metastasis. Radiother Oncol 2012;103:25-31. [Crossref] [PubMed]
- Nichols RC Jr, George TJ, Zaiden RA Jr, et al. Proton therapy with concomitant capecitabine for pancreatic and ampullary cancers is associated with a low incidence of gastrointestinal toxicity. Acta Oncol 2013;52:498-505. [Crossref] [PubMed]
- Sachsman S, Nichols RC Jr, Morris CG, et al. Proton therapy and concomitant capecitabine for non-metastatic unresectable pancreatic adenocarcinoma. Int J Part Ther 2014;1:692-701. [Crossref]
- Shinoto M, Yamada S, Terashima K, et al. Carbon ion radiation therapy with concurrent gemcitabine for patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2016;95:498-504. [Crossref] [PubMed]
- Khashab MA, Kim KJ, Tryggestad EJ, et al. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc 2012;76:962-71. [Crossref] [PubMed]
- Taniguchi CM, Murphy JD, Eclov N, et al. Dosimetric analysis of organs at risk during expiratory gating in stereotactic body radiation therapy for pancreatic cancer. Int J Radiat Oncol Biol Phys 2013;85:1090-5. [Crossref] [PubMed]
- Moningi S, Dholakia AS, Raman SP, et al. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol 2015;22:2352-8. [Crossref] [PubMed]
- Scorsetti M, Alongi F, Castiglioni S, et al. Feasibility and early clinical assessment of flattening filter free (FFF) based stereotactic body radiotherapy (SBRT) treatments. Radiat Oncol 2011;6:113. [Crossref] [PubMed]
- Cao M, Lasley FD, Das IJ, et al. Evaluation of rotational errors in treatment setup of stereotactic body radiation therapy of liver cancer. Int J Radiat Oncol Biol Phys 2012;84:e435-40. [Crossref] [PubMed]
- Lamb J, Cao M, Kishan A, et al. Online Adaptive Radiation Therapy: Implementation of a New Process of Care. Cureus 2017;9:e1618 [PubMed]
- Henke L, Kashani R, Robinson C, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol 2018;126:519-26. [Crossref] [PubMed]
- Fischer-Valuck BW, Henke L, Green O, et al. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image-guided radiation therapy system. Adv Radiat Oncol 2017;2:485-93. [Crossref] [PubMed]
- Mittauer K, Rosenberg S, Geurts M, et al. TU-AB-BRA-11: Indications for Online Adaptive Radiotherapy Based On Dosimetric Consequences of Interfractional Pancreas-To-Duodenum Motion in MRI-Guided Pancreatic Radiotherapy. Med Phys 2016;43:3735-6. [Crossref]
- Henke L, Kashani R, Yang D, et al. Simulated Online Adaptive Magnetic Resonance-Guided Stereotactic Body Radiation Therapy for the Treatment of Oligometastatic Disease of the Abdomen and Central Thorax: Characterization of Potential Advantages. Int J Radiat Oncol Biol Phys 2016;96:1078-86. [Crossref] [PubMed]
- Rudra S, Jiang N, Rosenberg S, et al. High dose adaptive MRI guided radiation therapy improves overall survival of inoperable pancreatic cancer. Int J Radiat Oncol Biol Phys 2017;99:E184 [Crossref]
- Crane CH. Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer. J Radiat Res 2016;57:i53-7. [Crossref] [PubMed]
- Crane CH, O’reilly EM. Ablative Radiotherapy Doses for Locally Advanced: Pancreatic Cancer (LAPC). Cancer J 2017;23:350-4. [Crossref] [PubMed]
- Krishnan S, Chadha AS, Suh Y, et al. Focal radiation therapy dose escalation improves overall survival in locally advanced pancreatic cancer patients receiving induction chemotherapy and consolidative chemoradiation. Int J Radiat Oncol Biol Phys 2016;94:755-65. [Crossref] [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 2010;304:1073-81. [Crossref] [PubMed]
- Van Laethem JL, Hammel P, Mornex F, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol 2010;28:4450-6. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704—a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 2012;82:809-16. [Crossref] [PubMed]
- Tepper J, Nardi G, Sutt H. Carcinoma of the pancreas: review of MGH experience from 1963 to 1973. Analysis of surgical failure and implications for radiation therapy. Cancer 1976;37:1519-24. [Crossref] [PubMed]
- Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer 1990;66:56-61. [Crossref] [PubMed]
- Van den Broeck A, Sergeant G, Ectors N, et al. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol 2009;35:600-4. [Crossref] [PubMed]
- Dholakia AS, Kumar R, Raman SP, et al. Mapping patterns of local recurrence after pancreaticoduodenectomy for pancreatic adenocarcinoma: a new approach to adjuvant radiation field design. Int J Radiat Oncol Biol Phys 2013;87:1007-15. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Radiation Therapy Oncology Group. RTOG 0848 protocol information, 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01013649
- Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg 2000;179:367-71. [Crossref] [PubMed]
- Picozzi VJ, Abrams RA, Decker PA, et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b–based chemoradiation: ACOSOG Trial Z05031. Ann Oncol 2011;22:348-54. [Crossref] [PubMed]
- Linehan DC, Tan MC, Strasberg SM, et al. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a single-institution phase II study. Ann Surg 2008;248:145-51. [Crossref] [PubMed]
- Allen AM, Zalupski MM, Robertson JM, et al. Adjuvant therapy in pancreatic cancer: phase I trial of radiation dose escalation with concurrent full-dose gemcitabine. Int J Radiat Oncol Biol Phys 2004;59:1461-7. [Crossref] [PubMed]
- Ma WW, Herman JM, Jimeno A, et al. A tolerability and pharmacokinetic study of adjuvant erlotinib and capecitabine with concurrent radiation in resected pancreatic cancer. Transl Oncol 2010;3:373-9. [Crossref] [PubMed]
- Wedén S, Klemp M, Gladhaug IP, et al. Long-term follow‐up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int J Cancer 2011;128:1120-8. [Crossref] [PubMed]
- Bernhardt SL, Gjertsen MK, Trachsel S, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer 2006;95:1474-82. [Crossref] [PubMed]
- Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011;253:328-35. [Crossref] [PubMed]
- Herman JM, Parkinson R, Onners B, et al. Preliminary results of a pilot study evaluating an allogeneic GM-CSF pancreatic tumor cell vaccine (GVAX) and cytoxan (cy) with stereotactic body radiation therapy (SBRT) and folfirinox (FFX) in patients with resected pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2015;93:S154. [Crossref]
- Goodman KA, Regine WF, Dawson LA, et al. Radiation Therapy Oncology Group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. Int J Radiat Oncol Biol Phys 2012;83:901-8. [Crossref] [PubMed]
- Jabbour SK, Hashem SA, Bosch W, et al. Upper abdominal normal organ contouring guidelines and atlas: a Radiation Therapy Oncology Group consensus. Pract Radiat Oncol 2014;4:82-9. [Crossref] [PubMed]
- Redmond KJ, Wolfgang CL, Sugar EA, et al. Adjuvant chemoradiation therapy for adenocarcinoma of the distal pancreas. Ann Surg Oncol 2010;17:3112-9. [Crossref] [PubMed]
- Rwigema JC, Heron DE, Parikh SD, et al. Adjuvant stereotactic body radiotherapy for resected pancreatic adenocarcinoma with close or positive margins. J Gastrointest Cancer 2012;43:70-6. [Crossref] [PubMed]
Cite this article as: Narang AK. Technical aspects of modern radiation therapy for pancreatic adenocarcinoma: field design, motion management, dosing, and concurrent therapy. Ann Pancreat Cancer 2018;1:21.

