Position emission tomography imaging in pancreatic cancer: recent progress and future directions
Introduction
Pancreatic cancer (PC) continues to have a dismal prognosis with an overall 5-year survival rate of about 8% (1). However, if the pancreatic tumor is confined to the primary location with surgical resectability at the time of diagnosis, survival could be significantly improved, with up to 26% of patients surviving more than 5 years (2). Late diagnosis contributes to poor prognosis. Unfortunately, due to the late onset of clinical symptoms, PC is sometimes diagnosed at advanced stages. Finding a feasible way to detect the disease during its initial stage is crucial.
In current clinical practice, the cornerstone of standardized diagnostic work-up for PC before surgery is the conventional radiological approach, such as computed tomography (CT) and magnetic resonance imaging (MRI). Endoscopic ultrasound (EUS) is used only occasionally. These imaging techniques detect tumors largely depending on anatomical or structural changes, which provide limited information and sometimes result in delayed diagnosis or misdiagnosis. In contrast to traditional imaging, position emission tomography (PET) enables clinicians to observe and monitor the functional, biochemical, and molecular characteristics of the tumor as well as the whole human body.
As a high-throughput tool, PET/CT provides a global snapshot of cellular physiology, biochemistry, and other activities, allowing the parallel assessment of thousands of metabolic products and biomarker expression. The aim of this review is to outline recent PET developments for PC and to discuss its future applications.
Applications of PET
Functional imaging as a surrogate for molecular assessment of tumor staging and diagnosis
Biomolecular information gained from PET scanning serves as a supplement to the standard tumor staging and diagnostic procedures, and could improve accuracy. PET is a noninvasive imaging technique, permitting repeated and serial scanning of lesion(s) with few side effects. This approach could help clinicians to differentiate malignant tumors from benign lesions during the wait-and-watch phase. This approach has been verified in tumors ranging from lung cancer to gastrointestinal tumors. Since the early 90s, PET imaging has been applied to the differential diagnosis of PC, particularly equivocal lesions.
Although the usefulness of PET has been reported, misdiagnoses occasionally occur in clinical practice when only simple semiquantitative data analysis of fluorodeoxyglucose (FDG) uptake is conducted. Compared with routine three-dimensional (3D) PET, four-dimensional (4D), PET could reduce the respiratory motion artifacts of tumors and obtain a significantly higher maximum standard uptake value (SUVmax), which helps facilitate clinical diagnosis. Yukutake et al. used 4D-PET to evaluate 36 patients with PC and reported a median SUVmax of 8.1±2.5 in 4D-PET and 6.2±2.1 in 3D-PET, respectively (P<0.01) (3). Similar results have also been generated by others (4,5). Additional approaches, such as introduction of novel radiotracers, contrast-enhanced techniques, and imaging integrations, would strengthen the role of PET in the diagnosis of PC.
Functional imaging as a guideline for treatment
Limited treatment options are available for patients with PC. Identifying the most feasible treatment regimen for a given patient or ensuring that patients who are most likely to benefit from specific therapies is important. PET radiolabeled with different tracers has been considered as a promising early imaging biomarker to assess treatment response.
Assessment of tumor resectability
To date, surgery remains the only option for radical cure for PC. The amazingly high mortality of this group of patients is partly due to the lack of surgical opportunity, which highlights the indispensability of proper tumor assessment and selection of the population that is most likely to benefit. PET may serve as an excellent, efficacious option to detect early-stage PC with high sensitivity/specificity, and thus permit more patients to receive radical surgery (6-11) (Table 1). In contrast, unnecessary surgical procedures could be avoided for patients who have locally advanced disease, nodal/distant organ metastases, and/or the presence of minimal hidden tumor spread.
Table 1
| Technique | Sensitivity (%) | Specificity (%) | Accuracy (%) | Reference |
|---|---|---|---|---|
| CT | 79.6–94 | 44.4–90 | −76.5 | (6) |
| MRI | 93 | 87 | 93 | (6,7) |
| 18FDG PET | 78–96.8 | 50–87 | 64–95.1 | (6,8,9) |
| 18FDG PET/CT | 85–97 | 61–94 | 85–95 | (6,8,9) |
| Enhanced PET/CT | 96 | 66.6 | 90.3 | (6,10) |
| Dual-phase 18FDG | 93 | 81 | 88 | (6,11) |
CT, computed tomography; MRI, magnetic resonance imaging; FDG, fluorodeoxyglucose; PET, position emission tomography.
Radiotherapy dose painting
Although no trials found survival benefit for patients with locally advanced PC receiving chemoradiotherapy (CRT) after induction treatment compared with chemotherapy alone, the first failure rate of nearly 30% in primary tumors indicated that the regimen of radiotherapy dose-escalation might be worthwhile to confer better local control (12). The high radioactive toxicity of surrounding normal tissues might limit its application. Similar to other abdominal organs, the pancreas exhibits high mobility. The mean intrafractional motion of the pancreatic head and tail in the supine/prone position were up to 12.8/8.9 and 13.0/10.0 mm, respectively, according to analysis of 4D computed tomography imaging data (13). Considering the large magnitude of respiratory motion and intrinsic radio-resistance of PC, definite target volume delineation is of great importance to spare normal organs and facilitate dose escalation with concurrent chemotherapy or other treatment modalities. The 1-to-2-cm margins that are commonly used to account for pancreatic tumor motion, based on conventional techniques, sometimes lead to excessive irradiation of nearby organs or normal tissues (14). The integration of PET functional imaging has provided more biomolecular information for radiotherapy treatment planning, in addition to the conventional anatomical changes. Recent reports have confirmed the potential of 4D-PET in precision targeting with more accurate generation of internal target volumes (ITV) in FDG-avid pancreatic tumors and helping to further individualize the radiotherapy plan. Professor Kishi has ever compared the ITV3D which was contoured using conventional respiratory un-gated PET with The ITV 4D which was contoured using 4D-PET. The final results showed that the ITV3D values were 2.0 (range, 1.1–3.4) fold larger than the corresponding ITV4D values (5). However, the appropriateness of radiotherapy dose painting based on PET imaging needs more examination.
Chemotherapy and targeted agent selection
Although much progress has been achieved with the addition of various new drugs, there has been no major breakthrough in therapeutic efficacy. The mainstay of chemotherapy for PC is still standard cytotoxic drugs. Gemcitabine has been established as the standard of care for inoperable and pre-/post-surgery treatment for PC patients with locally advanced tumors, metastases, suspicious surgical margins, and relatively higher performance status scores. Unfortunately, some patients with PC exhibit resistance to gemcitabine, which might result from low tumor drug uptake. Detecting the delivery of drug to tumors and enhancing the local drug concentration are crucial. Considering the similar uptake mechanism in tumors, researchers have used fluorothymidine (FLT) with F-18 radiotracer as a surrogate for gemcitabine. Excellent correlation was observed between FLT uptake level and treatment response to gemcitabine in vitro, indicating the potential of FLT PET to detect the population who might best benefit from gemcitabine and help patients who would not benefit from gemcitabine to avoid unnecessary treatment. Another gemcitabine analog, 1-(2’deoxy-2’fluoroarbinofuranosyl) cytosine (FAC), labeled with F-18 has demonstrated in vivo uptake in line with that of gemcitabine, suggesting its potential as a surrogate to monitor the intra-tumor drug uptake and distribution (15).
Since the invention of targeted agents, treatment toxicities have dramatically decreased, but only a modest survival benefit has been acquired. Although the interesting combination regimen of gemcitabine plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody erlotinib was confirmed by an international phase III trial to meet the primary end point, the relatively small actual survival benefit of a 2-week increases in the median overall survival time failed to reach a best cost-effect outcome. This finding might be due to the obscure expression status of about 70% of the patients included in this study (16). Considering the close relationship between EGFR mutation status and the efficacy of anti-EGFR inhibitors in other tumors like non-small cell lung cancer (NSCLC), the presence of EGFR mutations or EGFR amplification might also play an important role as the key biomarker during the selection of the population that might receive the greatest benefit from treatment. Thus, PET labeled with a specific radiotracer targeted against EGFR might be helpful during patient screening (17).
Immunotherapy selection
Immunotherapy has produced remarkable achievements in different obstinate malignancies, including refractory NSCLC, gastrointestinal tumors, advanced melanoma, and renal cell cancer. PC, as one of the leading cause of cancer-related death with limited treatment options, may also benefit from immunological therapies (18). Among these therapies, blockade of programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) pathway has emerged as a promising target for immune modulation in PC. Accumulating evidence demonstrated that inhibiting PD-1/PD-L1 interaction could reduce the growth rate of pancreatic tumors and decrease metastases, although no objective response has been observed among patients with PC (19,20). The strong contrast between the present explosive advancements in immunotherapy and the consistently poor response rate has further stimulated the development of a standardized procedure to select possible candidates for this intervention. Non-invasive, real-time molecular imaging of tumor PD-1/PD-L1 expression with PET using radiolabeled anti-PD-1/PD-L1 antibodies might play a role in accurate detection of PD-1/PD-L1 expression and accessibility. Thus far, this hypothesis has been verified in animal models. The encouraging results warrant more research to explore the rationality of this approach in clinical settings.
Functional imaging as a surrogate to predict prognosis and detect recurrence
Only limited therapeutic options are available for patients with PC. Therefore, there is an urgent need for effective prognostic assessment methods options for the timely evaluation of tumor response, which could help minimize patient exposure to possibly useless toxic therapeutic measures. Tumor size measurement based on Response Evaluation Criteria in Solid Tumors (RECIST) utilizing radiographic imaging has been generally considered a standard to evaluate treatment efficacy, but little information about tumor activities or pathologic response can be obtained by these conventional modalities (21). The SUV changes extracted from quantitative analysis of repeated PET imaging have been verified to serve as an effective prognostic indicator. Correlations between tumor pathological response and FDG uptake before and after preoperative CRT was observed in one study. Patients with PC who showed a high proportional alteration of SUV decline and a high pre-treatment SUV tended to experience a better pathological response after CRT (22). Another study about FLT PET reported that an increase in SUVmax at 60 min between the baseline and post-treatment FLT PET/CT scanning suggested futility of therapy, which could give these patients enough time and reasonable performance status to transfer to another, potentially effective treatment (23).
Timely salvage treatment after first relapse has been demonstrated to contribute to better survival and higher quality of life (QOL) in various cancers, including PC. Paralleling the diagnostic limitations, it is difficult to detect tumor recurrence in time. FDG-PET shows promise in distinguishing residual/recurrent lesions from post treatment changes in PC (24). However, the scanning time point of FDG PET/CT is important due to the artificial equivocal FDG uptake increase of radiation-induced inflammatory changes in the surrounding normal tissues. Without the inherent limitation of probable misinterpretation in FDG PET imaging, FLT PET seems to have a far superior capacity to detect recurrence, and thus may be more tumor-selective (25).
Cost-saving effect
Some researchers have questioned the value of PET in PC diagnosis from the view point of low cost/benefit ratio, that is, the relatively poor prognosis of PC compared with the correspondingly high cost of PET scan. A series of studies have confirmed that PET could be used to accurately determine the stage of tumor and evaluate its resectability, which is crucial for the next-step management, treatment cost, and QOL in patients with PC. In Japan, Higashi et al. found that PET detected that 35 cases (38%) were inoperable for various reasons, including peritoneal implantation metastases, distant lymph node metastases, liver or other organ metastases, and coexisting tumors (26).
Moreover, a deep tie exists between the pre-treatment SUV and postoperative prognosis in patients with PC (22). According to Yamamoto et al., patients with SUVmax ≥6.0 before surgery were more likely to experience poor postoperative survival because of the relatively high probability of microscopic venous infiltration in surgical specimens and high incidence of liver metastasis as a first site failure (27). Hence, for patients with low preoperative SUVmax who are predicted to be long-time survivors, physicians should attempt to alleviate all suspicious micro-metastases during surgery and strengthen the post-operative treatment to achieve radical eradication of tumor cells, while for patients with high SUVmax, more resource-intensive medical resources, including high-grade examinations, radical surgical procedures, or aggressive adjuvant treatments, should be avoided due to the lack of possible survival benefit (28). The SUV changes could also help avoid futile treatment and save more time and money for more therapeutic options (22). Based on the results from 59 patients with suspected PC, $188,500 could be saved by avoiding five pancreatic resections because of metastasis diagnosed by PET/CT. A reported amount of $1,066 per patient was saved by additional use of PET/CT (29).
Types of PET imaging modalities based on various mechanisms
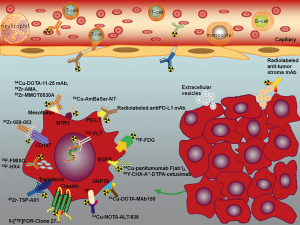
Clearly distinct from normal pancreatic cells, tumor cells exhibit disparate metabolic activities and proliferation in this resource-poor setting with obvious deprivation of nutrients, raw materials, and oxygen. Several studies made use of these specific characteristics to develop innovative diagnostic tools, including imaging examinations. Figure 1 and Table 2 outline the current potential molecular targets under investigation in PC.
Table 2
| Target | Radiotracers | Research level | Activity | Reference |
|---|---|---|---|---|
| Metabolism | ||||
| Glucose | 18F-FDG | Preclinical and clinical | Target expression for tumor diagnosis, staging, prognosis, resectability assessment, recurrence detection, treatment efficacy prediction, radiotherapy treatment planning | (5,18,20-35) |
| Hypoxia | 18F-FMISO | Preclinical and clinical | Target expression to assess the hypoxia in tumor, assessment of antiangiogenic treatment activity | (36-38) |
| 18F-HX4 | Preclinical and clinical | Target expression for radiation therapy planning, treatment response | (39) | |
| Amino acids transporter | ||||
| Choline | 18F-FEC | Preclinical | Target expression | (32) |
| Nucleotide synthesis | 18F-FLT | Preclinical and clinical | Target expression for tumor diagnosis, prognosis, recurrence detection; treatment response and toxicity; assessment of Pan-HER mAb mixture activity | (16,32,33,40-50) |
| Specific tumor cell biomarkers | ||||
| Cell surface protein | ||||
| GRP78 | 64Cu-DOTA-MAb159 | Target expression for evaluation of disease course and therapeutic efficacy | (51) | |
| Mesothelin | 64Cu-DOTA-11-25 mAb | Preclinical | Target expression | (52) |
| 89Zr-AMA | Preclinical | Target expression and distribution | (53) | |
| 89Zr-MMOT0530A | Clinical | Target expression, drug delivery and biodistribution | (54) | |
| NTR1 | 64Cu-AmBaSar-NT | Preclinical | Target expression | |
| Transmembrane protein | ||||
| Tissue factor | 64Cu-NOTA-ALT-836 | Preclinical | Target expression | (55) |
| Transferrin | 89Zr-TSP-A01 | Preclinical | Target expression, biodistribution of anti-transferrin receptor antibody | (56) |
| CD147 | 89Zr-059-053 | Preclinical | Target expression, biodistribution of anti-CD147 agent | (57) |
| Claudin | 5-[18F]FDR-C-CPE-17-KKK, 5-[18F]FDR-M19, 5-[18F]FDR-CC4P-5, 5-[18F]FDR-Clone 27 | Preclinical | Target expression for tumor diagnosis | (58) |
| Signaling pathway | ||||
| EGFR | 64Cu-panitumumab F(ab')2 | Preclinical | Target expression, assessment of panitumumab activity | (11) |
| 86Y-CHX-A”-DTPA-cetuximab | Preclinical | Target expression, assessment of cetuximab activity, radioimmunotherapy | (59) | |
| IGF1R | 89Zr-Df-1A2G11 | Preclinical | Target expression, biodistribution of antibody | (60) |
| Others | ||||
| Immune checkpoint | ||||
| PD-1/PD-L1 | NA | NA | NA | NIH5R01CA174294-03 |
| CTLA-4 | NA | NA | NA | NIH5R01CA174294-03 |
| Tumor stroma | ||||
| VEGFR | NA | NA | NA | (61) |
| MMPs | NA | NA | NA | (61) |
| Drug uptake | ||||
| Gemcitabine | 14C-gemcitabin,18F-FAC | Preclinical | drug delivery | (9) |
| Extracellular vesicles | ||||
| Proteins, mRNAs, miRNAs, DNAs | NA | NA | NA | (62-65) |
FDG, fluorodeoxyglucose; FMISO, fluoromisonidazole ; HX4, 3-Fluoro-2-(4-((2-nitro-1H-imidazol-1-yl) methyl)-1H-1, 2, 3-triazol-1-yl) propan-1-ol; FEC, fluorethylcholine; FLT, fluorathymidine; AMA, anti-mesothelin antibody; NTR1, neurotensin receptor 1; FDR, fluoro-5-deoxyribose; EGFR, epidermal growth factor receptor; IGF1R, insulin-like growth factor 1; PD-1/PD-L1, programmed death-1/programmed death-ligand 1; CTLA-4, cytotoxic T-lymphocyte associated protein 4; VEGFR, vascular endothelial growth factor receptor; MMPs, matrix metalloproteinases; NA, not available.
Metabolism-targeted PET imaging
Researchers have demonstrated the altered metabolic profiles in tumors, which might be utilized to make PET imaging agents with excellent signal-to-noise ratios. With growing insight into sophisticated mechanisms involved in the tumorigenesis, progression, and metastases of tumor cells, great progress has been achieved in metabolism-targeted imaging techniques. Among these, PET imaging targeting various molecules within different metabolic pathways, biochemical adaptions, or specific biomarkers has been pursued with the use of different tracers.
Metabolic PET imaging based on glucose metabolism
PET imaging based on the increased glucose metabolism of various tumor cells with a fluorine-18 fluorodeoxyglucose (18F-FDG) tracer has been the most widely used functional imaging modality in clinical practice. FDG is a glucose analog that can be phosphorylated smoothly, but cannot be metabolized further due to the lack of a 2’hydroxyl group, resulting in FDG accumulation in tumor cells with high metabolism. Numerous studies have been conducted to verify the role of 18F-FDG PET in diagnosis, therapeutic effect evaluation, efficacy monitoring, and recurrence detection in PC (5,8,9,26,28-35,40-42). The relatively broad overlap of FDG uptake between malignant tumors and inflammatory lesions might confuse clinicians, especially after tumors have been irradiated. With regard to this limitation, researchers have focused on designing effective methods to improve the diagnostic accuracy. An additional delayed scan was shown to be helpful, because the malignancies sometimes show a constantly stable SUV increase, while the FDG uptakes of most inflammatory lesions decrease over time (43). However, the feasible time interval between the first and second PET scan has been unclear. In addition, the cut-off SUV value to distinguish malignant tumors from benign lesions is not definitive, due to the huge variations in different PET institutions.
Some researchers hold the view that, for PC patients associated with elevated glucose levels, FDG PET scans could only be performed when their glucose levels recover to normal status after being prepared with insulin (33,44). Conversely, other studies confirmed no significant effect of blood glucose level on FDG uptake in patients with PC (32). One study conducted by Torizuka et al. using their in vitro human adenocarcinoma cell model suggested that acute hyperglycemia, but not chronic hyperglycemia, could significantly change FDG uptake (45). Based on the data published to date, there are no evident linear correlations between glucose levels and FDG uptake. Considering the complicated and probably indirect effects of glucose level on FDG uptake, researchers are attempting to make use of “glycemia-modified SUV” to help diagnose PC. Perhaps factors other than glucose level should be included in future analyses.
Metabolic PET imaging based on nucleotide synthesis
Analysis of 3’-deoxy-3’-18F-fluorothymidine (FLT) uptake as a surrogate marker of nucleotide synthesis that reflects the proliferative activity of cells has been studied to verify its feasible applications in tumor diagnosis, treatment monitoring, therapeutic prediction, and recurrence detection. Use of FLT PET before, during, or after treatment in PC is also being explored (23,36,41,42,46-50,66-68). One visual analysis of FLT PET imaging reported a sensitivity of 70% (23/33) and a specificity of 75% (6/8), indicating the clinical value of adding this approach to the diagnostic workup for PC (37). Another study published in 2015 reported that FLT uptake was promising as an early predictor of disease progression after gemcitabine-based chemotherapy in patients with advanced and metastatic PC (23). In line with the conclusions of other researchers, this study demonstrated that kinetic spatial filter (KSF) as a new temporal intensity information-based voxel-clustering approach in PET/CT could enable more accurate evaluation of treatment response, as well as clear visualization of liver metastases (39). In addition, FLT PET had no limitation of high susceptibility to inflammatory changes. It should be noted that gemcitabine influenced the FLT uptake level 24 h post treatment. FLT uptake is known to be mediated by hENT1; the uptake returned to baseline about 3 days later. Therefore, FLT PET scans should be performed at least 72 h after the treatment including gemcitabine. Considering the present controversies in the use of FLT PET in PC, more studies are needed.
Metabolic PET imaging based on hypoxia
Disruption of the equilibrium between proangiogenic and antiangiogenic factors in the tumor microenvironment leads to spawning of abnormal vessels. Vascular abnormality, characterized by impaired blood supply and interstitial hypertension, produces large tumor cell subpopulations that are poorly irrigated and hypoxic (38). Pancreatic tumors also characteristically have an oxygen-deficient microenvironment resulting from vascular abnormality and an abundance of stromal tissue, which partly accounts for their resistance to various therapies (51). Compared with well-oxygenated tumor cells, poor-oxygenated tumor cells exhibited low response to chemotherapy or radiotherapy (52). This makes hypoxia-targeted PET a priority to identify possible treatment-resistant sub-regions and help personalize treatment measures for patients with PC. The first pilot clinical study of 18F-fluoromisonidazole (FMISO)-PET did not detect a positive correlation between FMISO accumulation and tumor size, demonstrating minimal activity in PC tumors (53). One recently published preclinical study aimed to monitor vascular renormalization of tumor induced by antiangiogenic treatment with 18F-FMISO-avid PET, and found a significant SUV decrease in patient-derived pancreas xenografts (Panc286) after antiangiogenic dovitinib treatment (54).
Other hypoxia tracers, such as [18F]-3-Fluoro-2-(4-((2-nitro-1H-imidazol-1-yl) methyl)-1H-1, 2, 3-triazol-1-yl) propan-1-ol ([18F] HX4), are also being investigated for PET imaging in PC (59). Good repeatability was confirmed in both the size and location of high [18F] HX4 uptake regions according to repeated PET imaging, indicating that this compound might be a promising radiotracer for target delineation in radiotherapy. Furthermore, a series of studies found that the dynamics of hypoxic areas before and during treatment might be used to assess early response to particular therapies or to help predict prognosis (55). Thus, hypoxia-targeted PET imaging shows a potential to guide targeted anti-tumor treatment regimens, like dose painting of radiation according to hypoxia status, adding hypoxia-modifying agents for radio-sensitizing or other measures.
PET imaging targeting specific tumor cell biomarkers
A variety of upregulated receptors (cell surface protein or transmembrane protein) have been observed in PC cells, showing their potential to serve as the biomarkers to select tumor cells. Designing specific probes targeting these particular receptors in cancer cells remains the most promising strategy for molecular PET imaging. Researchers have endeavored to find the optimal biomarker that is highly expressed in PC or precursor pancreatic lesions and expressed at a low level in benign pancreatic lesions. Among the candidate receptors, several proteins have been investigated as targets for PET imaging in preclinical studies. Wang et al. conjugated 64Cu with chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) to the monoclonal antibody (Mab159) targeting GRP78, which is known as an upregulated cell surface protein in PC. Prominent tumor accumulation of 64Cu-DOTA-MAb159 was demonstrated in BXPC3 PC xenografts tumor model (56). Another highly expressed small glycoprotein—mesothelin was also explored as a novel target for PET imaging (57,58,60). Kobayashi et al. developed an anti-mesothelin antibody, known as 11-25 (57). The in vivo imaging showed a specific higher accumulation of radiotracer in subcutaneous xenograft tumor-bearing mice with two mesothelin-expressing PC cell lines (BxPC-3 and CFPAC-1), indicating the potential use of 64Cu-labeled 11-25 mAb as a PET probe in PC. Other antibodies targeting candidate receptors, such as anti-EGFR antibody (panitumumab, cetuximab) (17,69), anti-tissue factor antibody (ALT-836) (61), anti-transferrin receptor antibody (TSP-A01) (70), anti-CD147 antibody (059-053) (62), anti-claudin peptides (5-[18F]FDR-Clone 27) (63), anti-insulin-like growth factor antibody (Df-1A2G11) (64), and anti-neurotensin receptor antibody (65), are also being investigated in PC. The past decade has witnessed extensive progress in the examination of feasible biomarkers, but clinical translation of these modalities needs more optimization.
Other tracers
Several additional targets are currently being explored for the purposes of PET imaging. Recent efforts have centered on several immune checkpoint targets that are highly expressed on the tumor cell surface or in the microenvironment, with growing evidence supporting their crucial role in tumorigenesis, progression, dissemination, and metastases. The minimal progression of checkpoint inhibitors noted in PC studies seems to be attributable to the non-selectivity of the cases included in the studies. Thus, there is an urgent need to demonstrate and quantify the expression status and distribution of these molecules.
Immuno-PET employing different anti-checkpoint antibodies provides a new perspective for in vivo imaging of PC based on the uptake and distribution of a given tracer. Other potential targets under investigation are tumor stroma components, including vascular endothelial growth factor (VEGFR) and matrix metalloproteinases (MMPs) (71). Genetic mutations and altered gene expression patterns may also serve as targets for molecular imaging (71). In addition, active synthesis of protein and membrane lipids in proliferating tumor cells induces further demand for amino acids and choline, which show promise for radiolabeling as molecularly targeted radiopharmaceuticals for metabolic PET imaging (41,72). In-depth investigations into extracellular vesicles (EV) have identified their crucial role in PC progression, metastasis, cancer-related immunity, and treatment resistance. One 2015 study reported that the sensitivity and specificity of glypican-1 (GPC1)-positive circulating exosomes were 100% in diagnosing PC (73). Considering the relatively higher sensitivity, stability, and enrichment of EVs, which contain various specific tumor cell-associated molecules, including proteins, mRNAs, miRNAs, and DNA, extensive research has been conducted to explore their potential roles as novel biomarkers and therapeutic targets for PC (74-76). With increasing information about the molecular pathogenesis of PC, more novel imaging markers may be discovered and utilized to design more feasible tracers.
Limitations and progress direction
Lack of spatial resolution weakens the ability of PET to provide detailed anatomical information, like CT or MRI. Furthermore, for PC patients with peritoneal carcinomatosis, PET alone is insufficient to detect the diffuse infiltration with no obvious formations of nodules. Thus, more and more researchers focus on the development of specific software approaches or scanners to co-register the functional images of PET with anatomical images from CT, MRI, or other conventional techniques.
The combination of PET and CT makes it possible to evaluate anatomic and functional characteristics of tumor simultaneously. In addition, integrated PET/CT, especially enhanced PET/CT, could partly alleviate the shortcomings of PET in the detection of peritoneal carcinomatosis and infiltration of important vessels. One study conducted in Switzerland compared the diagnosis accuracy of different imaging modalities, and the results revealed a significant superiority of enhanced PET/CT to PET alone (P=0.035). Twelve patients (24%) with PET-demonstrated resectable tumors were judged to be unresectable by enhanced PET/CT due to locally advanced disease or distant metastases. Although the accuracy of PET was greatly enhanced through the specific fusion with enhanced CT imaging, nearly 10% of patients with enhanced PET/CT-demonstrated resectable tumors were ultimately shown to be surgically unresectable by laparotomy (31). Image co-registration and fusion combining MRI and PET were also explored, with no further radiation to the tumor entities, as is obviously the case for high-resolution CT. PET/MRI was helpful in the delineation of tumor uptake and its differentiation from surrounding tissue with pronounced physiologic tracer uptake, showing its feasibility for PET image interpretation correction (41).
Therefore, the so-called “one-stop-shop” or “all-in-one” protocol of enhanced multislice 18F-FDG PET/CT is still insufficient for preoperative disease staging (29,31). To overcome these limitations, apart from the fusion approach, more evaluation methods, such as routine laboratory tests (serum tumor marker CA19-9), physical examination, and histologic confirmation of suspected lesions through ultrasound-guided fine-needle aspiration cytology, laparoscopy or even exploratory laparotomy, should be added to improve the accuracy of diagnosis.
Several differences exist between the in vitro and in vivo metabolism of tumor cells. For PC tumor cells in culture, glutamine serves as the predominant carbon source for mitochondrial metabolism, while in vivo tumor cells are more dependent on glucose (77,78). This heterogeneity of both metabolism and biomolecular distribution in tumors would make the interpretation of PET imaging more confusing and challenging. In order to survive the nutrient/oxygen-replete conditions, the seemingly isolated tumor cell regions have acquired an ability to share metabolites and cross-feed each other, which could help fuel mutual growth. Considering the various factors affecting radiotracer uptake, analysis of PET imaging cannot be over-simplified and should not always be conducted following a similar protocol in different cases.
In conclusion, accumulating evidence has verified the important role of PET in PC, although numerous controversies remain. More imaging modalities of PET might be available with growing insight into various mechanisms involved in PC growth, invasion, and metastasis. More research is needed to demonstrate the feasibility and superior of PET in clinical settings.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yingbin Liu and Wei Gongi) for the series “The 8th Annual International Surgery Forum” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2019.03.02). The series “The 8th Annual International Surgery Forum” was commissioned by the editorial office without any funding or sponsorship. ML serves as an unpaid Associate Editor of Annals of Pancreatic Cancer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Wu AM. Engineered antibodies for molecular imaging of cancer. Methods 2014;65:139-47. [Crossref] [PubMed]
- Yukutake M, Sasaki T, Serikawa M, et al. The effect of respiratory-gated positron emission tomography/computed tomography in patients with pancreatic cancer. Hell J Nucl Med 2014;17:31-6. [PubMed]
- Kasuya T, Tateishi U, Suzuki K, et al. Role of respiratory-gated PET/CT for pancreatic tumors: a preliminary result. Eur J Radiol 2013;82:69-74. [Crossref] [PubMed]
- Kishi T, Matsuo Y, Nakamura A, et al. Comparative evaluation of respiratory-gated and ungated FDG-PET for target volume definition in radiotherapy treatment planning for pancreatic cancer. Radiother Oncol 2016;120:217-21. [Crossref] [PubMed]
- Wang XY, Yang F, Jin C, et al. Utility of PET/CT in diagnosis, staging, assessment of resectability and metabolic response of pancreatic cancer. World J Gastroenterol 2014;20:15580-9. [Crossref] [PubMed]
- Kim JK, Altun E, Elias J Jr, et al. Focal pancreatic mass: distinction of pancreatic cancer from chronic pancreatitis using gadolinium-enhanced 3D-gradient-echo MRI. J Magn Reson Imaging 2007;26:313-22. [Crossref] [PubMed]
- Rijkers AP, Valkema R, Duivenvoorden HJ, et al. Usefulness of F-18-fluorodeoxyglucose positron emission tomography to confirm suspected pancreatic cancer: a meta-analysis. Eur J Surg Oncol 2014;40:794-804. [Crossref] [PubMed]
- Tang S, Huang G, Liu J, et al. Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol 2011;78:142-50. [Crossref] [PubMed]
- Buchs NC, Buhler L, Bucher P, et al. Value of contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography in detection and presurgical assessment of pancreatic cancer: a prospective study. J Gastroenterol Hepatol 2011;26:657-62. [Crossref] [PubMed]
- Nishiyama Y, Yamamoto Y, Monden T, et al. Evaluation of delayed additional FDG PET imaging in patients with pancreatic tumour. Nucl Med Commun 2005;26:895-901. [Crossref] [PubMed]
- Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol 2011;29:3037-43. [Crossref] [PubMed]
- Kim YS, Park SH, Ahn SD, et al. Differences in abdominal organ movement between supine and prone positions measured using four-dimensional computed tomography. Radiother Oncol 2007;85:424-8. [Crossref] [PubMed]
- Feng M, Balter JM, Normolle D, et al. Characterization of pancreatic tumor motion using cine MRI: surrogates for tumor position should be used with caution. Int J Radiat Oncol Biol Phys 2009;74:884-91. [Crossref] [PubMed]
- Russell J, Pillarsetty N, Kramer RM, et al. In Vitro and In Vivo Comparison of Gemcitabine and the Gemcitabine Analog 1-(2'-deoxy-2'-fluoroarabinofuranosyl) Cytosine (FAC) in Human Orthotopic and Genetically Modified Mouse Pancreatic Cancer Models. Mol Imaging Biol 2017;19:885-92. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib Plus Gemcitabine Compared With Gemcitabine Alone in Patients With Advanced Pancreatic Cancer: A Phase III Trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Boyle AJ, Cao PJ, Hedley DW, et al. MicroPET/CT imaging of patient-derived pancreatic cancer xenografts implanted subcutaneously or orthotopically in NOD-scid mice using (64)Cu-NOTA-panitumumab F(ab')2 fragments. Nucl Med Biol 2015;42:71-7. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Zhao L, Li C, Liu F, et al. A blockade of PD-L1 produced antitumor and antimetastatic effects in an orthotopic mouse pancreatic cancer model via the PI3K/Akt/mTOR signaling pathway. Onco Targets Ther 2017;10:2115-26. [Crossref] [PubMed]
- Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007;13:2151-7. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Kittaka H, Takahashi H, Ohigashi H, et al. Role of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Predicting the Pathologic Response to Preoperative Chemoradiation Therapy in Patients With Resectable T3 Pancreatic Cancer. World J Surg 2013;37:169-78. [Crossref] [PubMed]
- Challapalli A, Barwick T, Pearson RA, et al. 3'-Deoxy-3'-(1)(8)F-fluorothymidine positron emission tomography as an early predictor of disease progression in patients with advanced and metastatic pancreatic cancer. Eur J Nucl Med Mol Imaging 2015;42:831-40. [Crossref] [PubMed]
- Daamen LA, Groot VP, Goense L, et al. The diagnostic performance of CT versus FDG PET-CT for the detection of recurrent pancreatic cancer: a systematic review and meta-analysis. Eur J Radiol 2018;106:128-36. [Crossref] [PubMed]
- van Waarde A, Jager PL, Ishiwata K, et al. Comparison of sigma-ligands and metabolic PET tracers for differentiating tumor from inflammation. J Nucl Med 2006;47:150-4. [PubMed]
- Higashi T, Saga T, Nakamoto Y, et al. Diagnosis of pancreatic cancer using fluorine-18 fluorodeoxyglucose positron emission tomography (FDG PET) --usefulness and limitations in "clinical reality". Ann Nucl Med 2003;17:261-79. [Crossref] [PubMed]
- Yamamoto T, Sugiura T, Mizuno T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol 2015;22:677-84. [Crossref] [PubMed]
- Wang Z, Chen JQ, Liu JL, et al. FDG-PET in diagnosis, staging and prognosis of pancreatic carcinoma: a meta-analysis. World J Gastroenterol 2013;19:4808-17. [Crossref] [PubMed]
- Heinrich S, Goerres GW, Schafer M, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005;242:235-43. [Crossref] [PubMed]
- Chong JU, Hwang HK, Lee JH, et al. Clinically determined type of 18F-fluoro-2-deoxyglucose uptake as an alternative prognostic marker in resectable pancreatic cancer. PLoS One 2017;12:e0172606 [Crossref] [PubMed]
- Strobel K, Heinrich S, Bhure U, et al. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer. J Nucl Med 2008;49:1408-13. [Crossref] [PubMed]
- Friess H, Langhans J, Ebert M, et al. Diagnosis of pancreatic cancer by 2[18F]-fluoro-2-deoxy-D-glucose positron emission tomography. Gut 1995;36:771-7. [Crossref] [PubMed]
- Diederichs CG, Staib L, Glatting G, et al. FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med 1998;39:1030-3. [PubMed]
- Akita H, Takahashi H, Ohigashi H, et al. FDG-PET predicts treatment efficacy and surgical outcome of pre-operative chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Eur J Surg Oncol 2017;43:1061-7. [Crossref] [PubMed]
- Sperti C, Pasquali C, Bissoli S, et al. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J Gastrointest Surg 2010;14:131-40. [Crossref] [PubMed]
- Paproski RJ, Young JD, Cass CE. Predicting gemcitabine transport and toxicity in human pancreatic cancer cell lines with the positron emission tomography tracer 3'-deoxy-3'-fluorothymidine. Biochem Pharmacol 2010;79:587-95. [Crossref] [PubMed]
- Herrmann K, Erkan M, Dobritz M, et al. Comparison of 3'-deoxy-3'-[(1)(8)F]fluorothymidine positron emission tomography (FLT PET) and FDG PET/CT for the detection and characterization of pancreatic tumours. Eur J Nucl Med Mol Imaging 2012;39:846-51. [Crossref] [PubMed]
- Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 2013;31:2205-18. [Crossref] [PubMed]
- Contractor K, Challapalli A, Tomasi G, et al. Imaging of cellular proliferation in liver metastasis by [18F]fluorothymidine positron emission tomography: effect of therapy. Phys Med Biol 2012;57:3419-33. [Crossref] [PubMed]
- Lachter J, Adler AC, Keidar Z, et al. FDG-PET/CT identifies a curable pancreatic cancer surgical tract metastasis after failure by other imaging modalities. Isr Med Assoc J 2008;10:243-4. [PubMed]
- von Forstner C, Egberts JH, Ammerpohl O, et al. Gene expression patterns and tumor uptake of 18F-FDG, 18F-FLT, and 18F-FEC in PET/MRI of an orthotopic mouse xenotransplantation model of pancreatic cancer. J Nucl Med 2008;49:1362-70. [Crossref] [PubMed]
- Nielsen CH, Jensen MM, Kristensen LK, et al. In vivo imaging of therapy response to a novel pan-HER antibody mixture using FDG and FLT positron emission tomography. Oncotarget 2015;6:37486-99. [Crossref] [PubMed]
- Nakamoto Y, Higashi T, Sakahara H, et al. Delayed (18)F-fluoro-2-deoxy-D-glucose positron emission tomography scan for differentiation between malignant and benign lesions in the pancreas. Cancer 2000;89:2547-54. [Crossref] [PubMed]
- Zimny M, Bares R, Fass J, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in the differential diagnosis of pancreatic carcinoma: a report of 106 cases. Eur J Nucl Med 1997;24:678-82. [Crossref] [PubMed]
- Torizuka T, Clavo AC, Wahl RL. Effect of hyperglycemia on in vitro tumor uptake of tritiated FDG, thymidine, L-methionine and L-leucine. J Nucl Med 1997;38:382-6. [PubMed]
- Lamarca A, Asselin MC, Manoharan P, et al. 18F-FLT PET imaging of cellular proliferation in pancreatic cancer. Crit Rev Oncol Hematol 2016;99:158-69. [Crossref] [PubMed]
- Debebe SA, Goryawala M, Adjouadi M, et al. 18F-FLT Positron Emission Tomography/Computed Tomography Imaging in Pancreatic Cancer: Determination of Tumor Proliferative Activity and Comparison with Glycolytic Activity as Measured by 18F-FDG Positron Emission Tomography/Computed Tomography Imaging. Mol Imaging Radionucl Ther 2016;25:32-8. [Crossref] [PubMed]
- Nakajo M, Kajiya Y, Tani A, et al. A pilot study of the diagnostic and prognostic values of FLT-PET/CT for pancreatic cancer: comparison with FDG-PET/CT. Abdom Radiol (NY) 2017;42:1210-21. [Crossref] [PubMed]
- Bollineni VR, Kramer GM, Jansma EP, et al. A systematic review on [(18)F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur J Cancer 2016;55:81-97. [Crossref] [PubMed]
- Seitz U, Wagner M, Neumaier B, et al. Evaluation of pyrimidine metabolising enzymes and in vitro uptake of 3'-[(18)F]fluoro-3'-deoxythymidine ([(18)F]FLT) in pancreatic cancer cell lines. Eur J Nucl Med Mol Imaging 2002;29:1174-81. [Crossref] [PubMed]
- Koong AC, Mehta VK, Le QT, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 2000;48:919-22. [Crossref] [PubMed]
- Rockwell S, Dobrucki IT, Kim EY, et al. Hypoxia and radiation therapy: Past history, ongoing research, and future promise. Curr Mol Med 2009;9:442-58. [Crossref] [PubMed]
- Segard T, Robins PD, Yusoff IF, et al. Detection of hypoxia with 18F-fluoromisonidazole (18F-FMISO) PET/CT in suspected or proven pancreatic cancer. Clin Nucl Med 2013;38:1-6. [Crossref] [PubMed]
- Hernández-Agudo E, Mondejar T, Soto-Montenegro ML, et al. Monitoring vascular normalization induced by antiangiogenic treatment with (18)F-fluoromisonidazole-PET. Mol Oncol 2016;10:704-18. [Crossref] [PubMed]
- Tachibana I, Nishimura Y, Shibata T, et al. A prospective clinical trial of tumor hypoxia imaging with 18F-fluoromisonidazole positron emission tomography and computed tomography (F-MISO PET/CT) before and during radiation therapy. J Radiat Res 2013;54:1078-84. [Crossref] [PubMed]
- Wang H, Li D, Liu S, et al. Small-Animal PET Imaging of Pancreatic Cancer Xenografts Using a 64Cu-Labeled Monoclonal Antibody, MAb159. J Nucl Med 2015;56:908-13. [Crossref] [PubMed]
- Kobayashi K, Sasaki T, Takenaka F, et al. A novel PET imaging using (6)(4)Cu-labeled monoclonal antibody against mesothelin commonly expressed on cancer cells. J Immunol Res 2015;2015:268172 [Crossref] [PubMed]
- ter Weele EJ, Terwisscha van Scheltinga AG, Kosterink JG, et al. Imaging the distribution of an antibody-drug conjugate constituent targeting mesothelin with (8)(9)Zr and IRDye 800CW in mice bearing human pancreatic tumor xenografts. Oncotarget 2015;6:42081-90. [Crossref] [PubMed]
- Klaassen R, Bennink RJ, van Tienhoven G, et al. Feasibility and repeatability of PET with the hypoxia tracer [(18)F]HX4 in oesophageal and pancreatic cancer. Radiother Oncol 2015;116:94-9. [Crossref] [PubMed]
- Lamberts LE. ImmunoPET with Anti-Mesothelin Antibody in Patients with Pancreatic and Ovarian Cancer before Anti-Mesothelin Antibody-Drug Conjugate Treatment. Clin Cancer Res 2016;22:1642-52. [Crossref] [PubMed]
- Hong H, Zhang Y, Nayak TR, et al. Immuno-PET of tissue factor in pancreatic cancer. J Nucl Med 2012;53:1748-54. [Crossref] [PubMed]
- Sugyo A, Tsuji AB, Sudo H, et al. Evaluation of (89)Zr-labeled human anti-CD147 monoclonal antibody as a positron emission tomography probe in a mouse model of pancreatic cancer. PLoS One 2013;8:e61230 [Crossref] [PubMed]
- Feni L, Omrane MA, Fischer M, et al. Convenient Preparation of (18)F-Labeled Peptide Probes for Potential Claudin-4 PET Imaging. Pharmaceuticals (Basel) 2017;10:99. [Crossref] [PubMed]
- England CG, Kamkaew A, Im HJ, et al. ImmunoPET Imaging of Insulin-Like Growth Factor 1 Receptor in a Subcutaneous Mouse Model of Pancreatic Cancer. Mol Pharm 2016;13:1958-66. [Crossref] [PubMed]
- Yin X, Wang M, Wang H, et al. Evaluation of neurotensin receptor 1 as a potential imaging target in pancreatic ductal adenocarcinoma. Amino Acids 2017;49:1325-35. [Crossref] [PubMed]
- Cieslak JA, Sibenaller ZA, Walsh SA, et al. Fluorine-18-Labeled Thymidine Positron Emission Tomography (FLT-PET) as an Index of Cell Proliferation after Pharmacological Ascorbate-Based Therapy. Radiat Res 2016;185:31-8. [Crossref] [PubMed]
- Herrmann K, Eckel F, Schmidt S, et al. In vivo characterization of proliferation for discriminating cancer from pancreatic pseudotumors. J Nucl Med 2008;49:1437-44. [Crossref] [PubMed]
- Quon A, Chang ST, Chin F, et al. Initial evaluation of 18F-fluorothymidine (FLT) PET/CT scanning for primary pancreatic cancer. Eur J Nucl Med Mol Imaging 2008;35:527-31. [Crossref] [PubMed]
- Nayak TK, Regino CA, Wong KJ, et al. PET imaging of HER1-expressing xenografts in mice with 86Y-CHX-A''-DTPA-cetuximab. Eur J Nucl Med Mol Imaging 2010;37:1368-76. [Crossref] [PubMed]
- Sugyo A, Tsuji AB, Sudo H, et al. Preclinical evaluation of (8)(9)Zr-labeled human antitransferrin receptor monoclonal antibody as a PET probe using a pancreatic cancer mouse model. Nucl Med Commun 2015;36:286-94. [Crossref] [PubMed]
- Cinar P, Tempero MA. Monoclonal antibodies and other targeted therapies for pancreatic cancer. Cancer J 2012;18:653-64. [Crossref] [PubMed]
- Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol 2011;38:55-69. [Crossref] [PubMed]
- Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177-82. [Crossref] [PubMed]
- Qiu J, Yang G, Feng M, et al. Extracellular vesicles as mediators of the progression and chemoresistance of pancreatic cancer and their potential clinical applications. Mol Cancer 2018;17:2. [Crossref] [PubMed]
- Lai X, Wang M, McElyea SD, et al. A microRNA signature in circulating exosomes is superior to exosomal glypican-1 levels for diagnosing pancreatic cancer. Cancer Lett 2017;393:86-93. [Crossref] [PubMed]
- Madhavan B, Yue S, Galli U, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer 2015;136:2616-27. [Crossref] [PubMed]
- Lyssiotis CA, Son J, Cantley LC, et al. Pancreatic cancers rely on a novel glutamine metabolism pathway to maintain redox balance. Cell Cycle 2013;12:1987-8. [Crossref] [PubMed]
- Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 2013;496:101-5. [Crossref] [PubMed]
Cite this article as: Zhang X, Meng X, Zhu H, Yang Q, Morris KT, Herman T, Yu J, Li M. Position emission tomography imaging in pancreatic cancer: recent progress and future directions. Ann Pancreat Cancer 2019;2:6.