
Multifunctional role of pancreatic stellate cells in pancreatic cancer
Epidemiology of pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) constitutes 90% of all pancreatic malignancies and is characterised by extensive desmoplasia and early metastasis. The risk factors for PDAC include a family history of the disease, cigarette smoking, chronic pancreatitis, obesity and diabetes mellitus (1,2). PDAC most commonly occurs in the 60 to 80 year age group, and its incidence is 50% higher in men than in women and (1) genetic mutations such as BRCA2, CDKN2A, STK11, PRSS1, SPINK1, CFTR, PALB2 and ATM increase the risk for PDAC (3), patients with chronic pancreatitis have a 15 fold increased risk of developing PDAC (4), cigarette smoking increases the risk of PDAC by 75% even up to 10 years after cessation of smoking and (5) diabetes mellitus increases the risk by 30% up to 20 years after diagnosis (6). Obesity and high body mass index are positively correlated with the risk of developing PDAC (7). One meta-analysis showed a 19% increase in risk of developing PDAC in obese individuals (BMI ≥30 kg/m2) compared to normal participants (BMI 22 kg/m2) (8).
The genetic mutational landscape of PDAC involves the alteration of 69 genes which affect 12 core signalling pathways (9). Both oncogenes and tumour suppressor genes are mutated of which the major genes involved in PDAC are the oncogene Kristen Rat Sarcoma Virus (KRAS), and three tumour suppressor genes—TP53, CDKN2A and SMAD4. KRAS is the earliest mutation seen in low-grade pancreatic intraepithelial neoplasia (PanIN) lesions, the precursors of PDAC (10). CDKN2A is also found early in neoplasia while SMAD4 and TP53 alterations occur late in tumorigenesis corresponding to grade 3 PanINs and invasive stages (11).
Despite its low incidence, PDAC is the fourth leading cause of cancer related deaths and, alarmingly is projected to become the second leading cause of cancer related deaths by 2030 (12). The high mortality in PDAC can be attributed to the presence of metastases even at the time of first diagnosis and the lack of effective treatments. Even with advances in current chemotherapy such as Folfirinox, and Gemcitabine-Abraxane, in addition to surgery and radiotherapy, the 5-year survival rate of PDAC has only marginally improved from 4% a decade ago to 8% (13,14). Surgical resection is the mainstay of treatment for prolonged survival (15) but unfortunately only 15–20% of cases are suitable for surgical resection because the majority of patients have visible metastatic disease at the time of diagnosis (16).
Role of stroma in pancreatic cancer
Desmoplasia is a striking feature of PDAC accounting for 50–80% of the tumour volume (Figure 1) (17). This stromal reaction consists of cellular components such as pancreatic stellate cells (PSCs), which are the main source of the stromal collagen (Figure 2) (18), fibroblasts, immune cells, vascular and neural elements and non-cellular components such as collagens, fibronectin, glycoproteins, proteoglycans, hyaluronic acid, cytokines, growth factors, and secreted protein acidic and rich in cysteine (SPARC) (19). The immune cells mostly comprise immunosuppressive leukocytes such as myeloid derived suppressor cells (MDSC) and macrophages (20).
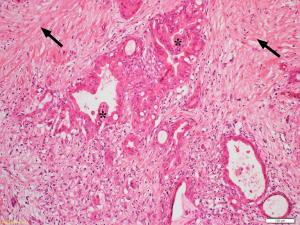
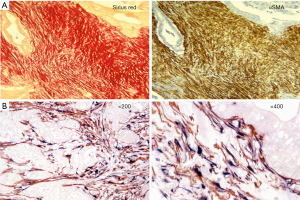
Several studies have examined the influence of the stroma on the progression and outcome of PDAC (21,22). In a cohort of 233 patients who underwent surgical resection, Erkan et al. (23) demonstrated that overt stromal activity, as assessed by the expression of alpha smooth muscle actin (αSMA, a marker of activated PSCs) was associated with a poor prognosis. Similarly, Fujita et al. (24) observed that in 109 patients with PDAC, higher αSMA mRNA levels were correlated with poorer outcome. A stage-dependent influence of stroma on PDAC outcome was observed by Wang et al. (25) in a study involving 145 PDAC patients who underwent resection followed by gemcitabine-chemotherapy. The authors reported that increased expression of αSMA was significantly associated with poor outcome only during the early stages of the tumour development. Higher expression of other stromal factors such as annexin II, stromal tenascin C (26), interleukin-1 receptor-associated kinase 4 (IRAK4) expression (27), SPARC (28) and periostin (29) have also been reported to correlate with poor prognosis. Thus, these studies support the concept that stromal activity correlates with the progression of PDAC and a poor outcome.
In contrast to the above findings, Ozdemir et al. (30) observed that low expression of αSMA correlated with worse outcome, albeit in a relatively small cohort of 53 PDAC patients. In addition, Bever et al. (31) observed that high stroma density was associated with longer survival but that stronger expression of αSMA was not associated with differences in clinical outcome.
The apparent contradictory findings on the influence of stroma on PDAC progression in the above studies could be due to the differences in the methodology of assessment of stromal activity (stromal density vs. αSMA expression), differences in patient cohorts, and stage of the tumour (early vs. late) and a possible biphasic influence of stroma on tumour progression. Another factor that may be relevant to the disparate findings is the presence of functionally different subsets of PSCs within the stroma of pancreatic tumours. In this regard Yuzawa et al. (32) have reported the presence of fibroblasts with varying expression of αSMA and PDGFRβ, with higher expression of PDGFRβ being associated with a worse prognosis, but αSMA being unrelated to outcome. In another study, Ohlund et al. (33) showed that the PSC-derived cancer-associated fibroblasts (CAFs) close to cancer cells exhibited increased αSMA expression, but those at the periphery of the tumour, (away from the tumour cells), lacked αSMA expression, secreted inflammatory mediators including IL-6 and were correlated with poor outcome. Overall, the weight of evidence supports the view that the stroma exerts significant influence on the progression of PDAC, suggesting a need for selectively targeting and reprogramming the stroma to help improve patient outcomes.
PSCs in the healthy pancreas
The finding by Apte et al. (18) that the collagenous stroma of the pancreas is produced by PSCs has led to numerous studies of the functions of this cell. We now know that in the normal pancreas, PSCs are stellate-shaped cells located around pancreatic acini and account for 4–7% of all parenchymal cells in the gland (34). In health, PSCs manifest a quiescent phenotype characterised by abundant vitamin A-containing lipid droplets in the cytoplasm. PSCs are identified by immunostaining for selective markers, such as desmin, glial fibrillary acidic protein (GFAP), nestin, neural cell adhesion molecule, nerve growth factor and synemin (35-37). Although the origin of PSCs is not clear, it was demonstrated that a proportion of PSCs originate from bone marrow and replenish PSCs in the pancreas (38). In addition, Ino et al. (39) showed that chemokine receptor type 2 (CCR2+) monocytes migrate from the bone marrow and transform into PSCs through the monocyte chemoattractant protein-1 (MCP-1)/CCR2 pathway. The monocyte lineage origin of PSC is also supported by the expression of monocyte specific marker α-naphthyl butyrate esterase (ANBE) (40).
PSCs play key roles in both the healthy and diseased pancreas. In normal pancreas, PSCs express Toll-like receptors (TLRs) 2, 3, 4 and 5 (41) and have the ability for phagocytosis (42) and play a part in innate immunity as first-line defense against early injury. PSCs also secrete matrix metalloproteinases such as MMP2, MMP9 and MMP13 and metalloproteinase inhibitors such as TIMP1 and TIMP2 to maintain the balance of extracellular matrix, which is usually disrupted after activation in pancreatitis and PDAC. Several in vitro and in vivo studies suggest that PSCs may have progenitor-like capabilities, possessing ATP-binding cassette G2 (ABCG2) and they transform into insulin-secreting cells upon exposure to the relevant culture medium (40,43-46).
Response of PSCs to injury
During pancreatic injury, PSCs are activated, a process associated with loss of the vitamin A-containing lipid droplets and transformation to a myofibroblast-like phenotype with expression of the activation marker alpha smooth muscle actin (αSMA), fibroblast activation protein-α (FAP-α), fibroblast-specific protein-1 (FSP-1), and fibrinogen (35). Apte et al. (17,37,47) were the first to demonstrate that the collagenous stroma in pancreatic injury was produced by PSCs. Platelet derived growth factor (PDGF) induces differentiation of PSCs into the myofibroblast phenotype (Figure 3) (32,48). In addition, activated PSCs express intercellular adhesion molecule (ICAM)-1, produce cytokines including interleukins 6, 7, and 8 and monocyte chemoattractant protein (MCP), and induce angiogenesis (34,49-51).
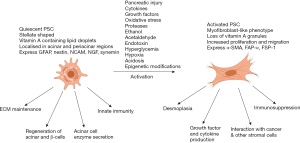
PSCs can be activated by several mediators such as pro-inflammatory cytokines (52), oxidant stress (53,54), ethanol and its metabolites, acetaldehyde and fatty acid ethyl esters (55,56), fatty acids (oleate) (57) and endotoxins (58). Other PSC activating factors of particular relevance to PDAC include acidosis (59), hypoxia (60), increased interstitial pressure (61), and hyperglycaemia (62). Recently, epigenetic modifications (increased acetylation of histones) have been shown to play an important role in the activation of PSCs and collagen synthesis (63). Bromodomain extra terminal domain (BET) proteins bind to acetylated motifs on histones leading to gene activation via recruitment of transcription factors and other chromatin regulators (64). Kumar et al. (63) demonstrated that BET proteins are expressed in PSCs and that pan inhibition of BET proteins (subtypes BRD 2, 3, and 4) using JQ1, a BET inhibitor leads to PSC quiescence and decreased collagen I synthesis. Furthermore, using specific siRNA for the BRD subtypes, the authors found that collagen synthesis was positively regulated by BRD4 but negatively regulated by BRD 2 and 3.
Mechanical properties of the local environment have been shown to play a role in activating PSCs. Using a physiomimetic system to recapitulate the mechanical microenvironment, Lachowski et al. (65) demonstrated that matrix stiffness can regulate the activation and migration of PSCs. These findings implicate the role of mechanical stimulation by the microenvironment in the progression PDAC through activation of PSC and fibrosis.
Interactions of PSCs and cancer cells
Apte et al. (18) established that activated PSCs are primarily responsible for the characteristic desmoplasia in PDAC. Activation of PSCs is considered an early event in PDAC due to the presence of activated PSCs expressing periostin—a cell adhesion protein, galectin-1 (a glycan binding protein) and αSMA (an activation marker) in precursor lesions such as PanINs (66) and intra-ductal papillary mucinous neoplasms (IPMN) (67). It is now established that PSCs and tumour cells have bidirectional effects on each other. On one hand, pancreatic cancer cells (PCCs) promote PSC proliferation, migration and extracellular matrix synthesis (68,69) while on the other hand PSCs promote PCC progression, migration, and tumour cell survival (68,70).
The complementary bidirectional influence of PCCs and PSCs has been well demonstrated by both in vitro and in vivo studies. In co-culture studies, PCCs increased PSC proliferation and ECM synthesis mediated by PDGF, FGF2, and TGFβ1 (71). PCCs also promoted the secretion of matrix metalloproteinases by PSCs (72) through ECM metalloproteinase inducer (EMMPRIN) secretion (73) and TGFβ1 signalling (74). It is interesting to note that cancer cells induce autophagy in PSCs resulting in the degradation of proteins and release of amino acids such as alanine which then acts as an alternative carbon source for the TCA cycle and lipid synthesis in cancer cells. As a result, cancer cells have decreased dependency on glucose and glutamine in the nutrient-poor, and hypoxic environment of PDAC (75). The process of autophagy also activates PSCs leading to increased stromal synthesis. Endo et al. (76) demonstrated that knockdown of autophagy proteins in PSCs led to a decrease in ECM and IL-6 production in vitro and that co-administration of these PSCs with PCCs led to smaller tumours and fewer metastases in nude mice. The authors also observed that in 133 patient-derived PDAC samples, autophagy significantly co-related with tumour growth, invasion, metastases and poor outcome.
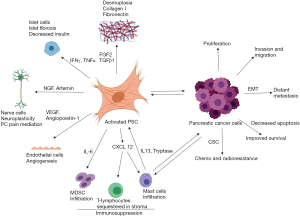
PSCs influence several critical steps in PDAC progression. PSCs induced epithelial mesenchymal transition (EMT) leading to increased invasion and migration of PCCs (77,78), and formation of a cancer stem cell (CSC) phenotype (79) (Figure 4). Although PSCs exhibit functionally diverse subsets with differential influence on PDAC progression (22), stromal signalling appears to be indispensable for PDAC progression. Indeed, Sherman et al. (80) clearly demonstrated that Kras mutation-induced signalling (the major gene mutation involved in PDAC) alone was insufficient to drive oncogene transcription and required permissive signalling from the stroma for the development of PDAC.
In animal models, co-injection of PCC with PSCs resulted in larger tumours with significant desmoplasia compared to injection of PCCs alone in both subcutaneous xenograft models (71) and orthotopic models (68,70,81); this increase in tumour size was due in part to PSC-induced PCC proliferation. The overexpression of the serine protease inhibitor SERPINE2 by PCCs was reported to be responsible for the increased tumour growth in vivo in the presence of PSCs (82).
PSCs also have a facilitatory role in metastasis of tumour cells. In a gender mismatch study, Xu et al. (69) demonstrated the presence of PSCs in metastatic nodules suggesting dissemination of PSCs via the circulation to distant organs, possibly facilitating the seeding and growth of cancer cells at these sites. Similar observations were made by Suetsugu et al. (83) who observed that both PSCs and PCCs co-migrated from the spleen (the site of injection), to the metastatic nodules. Storck et al. (84) demonstrated that calcium-gated potassium 3.1 channels are crucial for the process of migration in PSCs and blocking these channels abolished both migration and chemotaxis of PSCs. In addition to migrating PSCs, local hepatic stellate cells in the liver also aid the formation of a metastatic niche for migrating PCCs (85).
Recently, exosomes have emerged as important mediators of the bidirectional interactions between stellate cells and tumour cells. Takikawa et al. (86) demonstrated that exosomes derived from PSCs contained a variety of microRNAs and an abundance of miR-21-5p and miR-451a and mediated proliferation and migration of PCCs. Similarly, exosomes derived from PCCs stimulated the activation, proliferation, and migration of PSCs through upregulation of transforming growth factor β1 (TGFβ1) and tumour necrosis factor (TNF) (86). The signalling pathways involved in the PSC and PCC interactions are summarised in Table 1.
Table 1
| Signalling pathway | Mediator | Functional role | Inhibitor/activator used | Reference |
|---|---|---|---|---|
| Rho-ROCK pathway | Rho-associated protein kinase | α-SMA expression, proliferation, chemotaxis, type I collagen, fibrosis | Y-27632: a specific ROCK inhibitor | (87) |
| Mitogen-activated protein kinase (MAPK) signalling pathway | MAPK | PSC proliferation, TIMP-1 production, α-SMA | U0216: a specific MAP kinase (MEK) inhibitor | (88) |
| PI3-Kinase pathway | PDGF | PSC migration and proliferation | Wortmannin: a specific |
(89) |
| SMADS | SMAD-2,3 | PSC activation, proliferation, ECM deposition, transdifferentiation, TGF-β1 expression | PD98059: a specific inhibitor of mitogen-activated protein kinase (MEK1) | (90) |
| Protein kinase C | Hyperglycaemia | PSC proliferation, α-SMA, collagen-I production, angiogenesis | Calphostin C: a protein kinase C (PKC)-specific inhibitor | (91) |
| Peroxisome proliferator-activated gamma (PPARγ) | PPAR-γ ligands | Inhibition of PSC activation, proliferation, and collagen synthesis; increased phagocytosis | siRNA | (42) |
| Hedgehog pathway | Smo, Gli | PSC activation, ECM synthesis, migration, desmoplasia, angiogenesis; PCC proliferation, migration and chemoresistance | NVP-LDE225: a Hh pathway inhibitor | (92) |
| Wnt/β-catenin signalling | β-catenin | PSCs promote invasion of PCCs | Retinoic acid | (93) |
| Vitamin D receptor | Vitamin D | Inhibition of PSC activation; decreased chemoresistance | Calcipotriol: a vitamin D receptor activator | (94) |
| Toll-like receptor (TLR) signalling | TLR9 | Expression of PSC-derived cytokines (e.g., CCL3, CCL11) immunosuppression | ODN1826: a TLR9 ligand | (95) |
| Periostin pathway | Periostin | Periostin secreted by PSCs promotes PCC proliferation, EMT and resistance to nutrient deprivation and hypoxia | Erlotinib: an EGFR inhibitor and SCH772984: an Erk inhibitor | (96) |
| Hypoxia inducible factor 1 (HIF-1) | CCL2 | PSC activation, macrophage recruitment | HIF-1α siRNA | (97) |
| HGF/c-MET pathway | HGF | PSC promote proliferation and metastasis of tumour cells | AMG 102: monoclonal antibody for HGF; compound A: small molecule inhibitor of c-MET | (81) |
| IL6/JAK/STAT | IL-6 | PSC activation and proliferation | Ruxolitinib: a Jak1/2 inhibitor; MEK162: a MAPK inhibitor | (98) |
| Integrin | Kindlin-2 | Increased cytokines production in PSCs facilitating progression and migration of PCC | Kindlin-2-knockdown | (99) |
| Galectin-1 | PSC-derived SDF-1 | Proliferation of PSC and chemokine secretion facilitating PCC metastasis | BAY 11-7082: a NF-κB inhibitor; AMD3100: a CXCR4 blocker | (100) |
| Reactive oxygen species (ROS) | Suppression of miRNA-21 | PSC activation and induction of glycolysis | Resveratrol | (101) |
| CXCL12 (SDF-1) signalling | PSCs-derived CXCL12 (SDF-1) | Immunosuppression by preventing CD8+ T cells | AMD3100: an SDF-1/CXCL12 inhibitor | (102) |
α-SMA, alpha smooth muscle actin; c-MET, tyrosine-protein kinase of Met; CCL, chemokine ligand; CXCL, chemotactic cytokine ligand; ECM, extracellular matrix; JAK/STAT, Janus kinase/signal transducers and activators of transcription; HGF, hepatocyte growth factor; PSC, pancreatic stellate cells; PCC, pancreatic cancer cells; SDF, stromal derived factor; PDGF, platelet-derived growth factor; SMAD, small worm mothers against decapentaplegic.
Interactions of PSCs with other cells in the stroma
PSCs interact with other stromal cells such as endothelial cells, immune cells, neural cells and islet cells in the stroma. PSCs interact with endothelial cells by producing proangiogenic factors such as vascular endothelial growth factor (VEGF) (69), periostin (29), angiopoietin-1 (60), and Hepatocyte growth factor (HGF) (103) which are shown to induce endothelial proliferation in vitro. On the other hand, PSCs also display antiangiogenic features such as (I) secretion of collagenous stroma (104,105), (II) expression of vasohibin-1, which acts as negative feedback for VEGF-induced gene expression (60,106), and (III) induction of PCCs to secrete endostatin (104). Taken together, the bi-functional role of PSCs in angiogenesis may play a role in the selective vascularisation in PDAC, wherein tumours are usually better perfused at the periphery (leading edge) and less perfused towards the core (107). While pro-angiogenic factors expressed by PSCs are responsible for the hypervascularity, cancer cell-secreted endostatin is implicated in the hypo-vascularity of the juxta-tumoral stroma (108). Furthermore, targeting PSCs has been demonstrated to reduce neo-vascularisation in vivo (81).
PSCs are involved in immune modulation in PDAC. PSCs express TLRs 2, 3, 4 and 5 and take part in innate immunity (41). Notably, PSCs play an important role in creating an immunosuppressive environment via the secretion of proinflammatory cytokines which mediate a number of effects on immune cells including:
- Sequestration of cytotoxic CD8+ T-cells (thereby preventing them from acting on cancer cells), mediated by CXCL12 produced by PSCs;
- Infiltration of CD4+ Foxp3+ Tregs cells and reduced T-cell and NK-cell mediated cytolysis, associated with increased production of the chemokine IP-10;
- Elevated MDSC infiltration and differentiation of MDSCs to a CD11b+ CD33+ phenotype which suppress T-lymphocyte proliferation; the latter effect is induced by IL6 produced by PSCs;
- T cell apoptosis and Th2 cytokine secretion—effects mediated by galectin-1 which is overexpressed in PSCs;
- Mast cell infiltration (109) resulting from PSC mediated activation of mast cells which in turn further activate PSCs through IL-13 and tryptase.
As noted earlier, PSCs express the neural marker GFAP and secrete several neurotrophic factors such as nerve growth factor (NGF) and brain derived neurotropic factor (BDNF) (110,111). PSCs are implicated in the perineural invasion and migration of tumour cells along nerve axons (112) and may contribute to the pain of PDAC through the production of the neurotropic factor TRPV1 (111). These effects are mediated by activation of the sonic hedgehog pathway in both PCCs and PSCs.
As mentioned earlier, diabetes is a major risk factor for PDAC. The role of PSCs in islet cell dysfunction is suggested by the identification of activated PSCs in and around fibrosed islets in a diabetic rat model and by the findings that pancreatic β-cells co-cultured with PSCs exhibit decreased insulin expression and increased apoptosis (113). The role of PSCs in new onset diabetes (which has been recently recognised as an important predictive factor for PDAC development) is as yet unknown. While adrenomedullin a 52-amino acid peptide produced by PDAC cells has been implicated in PDAC associated new onset diabetes due to the ability of the peptide to inhibit insulin secretion (114), the influence of adrenomedullin on PSC function remains to be investigated.
Recently, the role of PSCs in ECM remodelling is gaining attention. Using an in vitro 3D spheroid tumour model and advanced imaging techniques, Drifka et al. (115) demonstrated the permissive role of PSCs in fashioning the alignment of collagen fibres to facilitate the invasion and co-migration of cancer cells through the stroma. Similarly, Sada et al. (116) also using a 3D culture setting, have reported that PSCs, in response to hypoxia, reorganised collagen fibres to a parallel alignment facilitating cancer cell migration. These interactions between PSCs and ECM are shown to be dependent on cluster of differentiation 51 (CD51) an integrin expressed in PSCs (117) which is also associated with aggressive disease and poor outcome. Other stromal proteins that are involved in PSC-ECM interactions include lumican, which enhances PSC adhesion and mobility (118) and periostin, which promotes cancer cell proliferation and metastasis (119).
PSCs and chemo and radio resistance
PDAC is highly resistant to chemotherapy as well as radiotherapy. Extensive desmoplasia combined with fibrosis mediated hypoxia leads to impaired drug delivery and chemoresistance (60,120,121). PSCs play an important role in the development of chemoresistance through various mechanisms. Activated PSCs secrete cytokines and large amounts of ECM rich in laminin, desmin, and collagen I/III (34) leading to fibrosis and decreased vascularity which hampers the translocation of drugs to cancer cells. When co-injected with cancer cells into mouse pancreas, PSC promote proliferation, migration, and reduced apoptosis of cancer cells, leading to large tumour volumes contributing to chemoresistance (68,70,122). Furthermore, PSCs are known to induce stemness in cancer cells which may also contribute to resistance of the cancer cells to chemotherapy (79). Stromal-derived factor-1 acting through the CXCR4 receptor was hypothesised to help in the evasion of gemcitabine-induced apoptosis of tumour cells in PDAC (123). PSCs are shown to increase the resistance of tumour cells to gemcitabine through increased expression of hairy and enhancer of split-1 (Hes1) in the notch signalling pathway (124).
A more recent report indicates that PSCs in primary tumours can internalise and store the active form of gemcitabine within their cytoplasm (due to low expression of inactivating enzymes), consequently preventing the drug from reaching tumour cells (125).
PSCs are also implicated in resistance of PDAC to radiotherapy. Mantoni et al. (126) demonstrated a radioprotective effect of PSCs on PCCs through β1-integrin signalling. On the other hand, PSCs also enhance the stem cell phenotype in cancer cells through EMT mediated by TGFβ leading to radiotherapy resistance (127).
Targeting stromal-tumour interactions
Despite the recent experimental advances in combinations of chemotherapeutic agents, the impact on the survival of patients with PDAC is disappointing. We assert that the failure of current treatments may be partly due to the fact that stromal-tumour interactions have largely been ignored in drug development efforts in the past. However, with the increasing recognition of the critical role of the stroma in PDAC progression, several studies targeting stromal-tumour interactions have recently been reported, with encouraging results in the pre-clinical phase, and with some being taken to early clinical trials. These are summarised as follows.
Hedgehog pathway
The hedgehog pathway in PDAC manifests as overexpression of the ligand sonic hedgehog (Shh) in tumour cells with overexpression of its receptor Smoothened (Smo) mainly in cancer-associated fibroblasts (128). In murine models, ormeloxifene (129) and IPI-926 (130) were successful in targeting the Hh pathway resulting in decreased stroma, reduced tumour volume and increased sensitivity to co-administered gemcitabine. Rucki et al. (92) used KPC mice to demonstrate that dual inhibition of sonic hedgehog (Hh) and HGF/c-MET pathway sensitises PDAC to gemcitabine. However, targeting the Hh pathway in a clinical trial GDC-0449 using the inhibitors vismodegib, saridegib and sonidegib failed to yield a significant survival benefit (131).
HGF/c-MET pathway
HGF and its receptor c-MET are increasingly recognised for their role in stromal-tumour interactions in PDAC. In PDAC, HGF is produced by PSC while its receptor is expressed on cancer cells and this pathway is involved in cancer cell proliferation, invasion and metastases (103,122). Recent studies report that inhibition of the HGF/c-Met pathway in an orthotopic mouse model developed by co-injection of cancer cells and PSC resulted in decreased tumour size and elimination of metastasis (81). Li et al. (132) observed that treatment with a combination of c-Met inhibitor XL184 and gemcitabine in a subcutaneous xenograft mouse model significantly decreased cancer growth, while individual treatments with either XL184 or gemcitabine were ineffective in decreasing the growth rate. Pothula et al. (81,122) showed that inhibition of both arms of HGF and c-Met pathway along with gemcitabine completely eliminated metastasis in an orthotopic mouse model strongly resembling human disease. The authors also found that gemcitabine alone stimulated stemness and aggressiveness of cancer cells suggesting the need for combination therapy. These findings have been corroborated by other studies which showed that gemcitabine treatment alone increased CSC numbers in PDAC (133,134).
A recent study suggests that c-MET downstream signalling is important for the development of an aggressive tumour phenotype. Lomberk et al. (135) examining epigenetic landscapes in PDAC showed that tumour populations can be grouped into two distinct epigenomic landscapes namely less aggressive “classical type” and more aggressive “basal type” tumours. Further, utilising super-enhancer mapping coupled with transcription factor binding motif and upstream regulatory analyses, the authors demonstrated that downstream MET signalling is involved in tumour proliferation and EMT in “basal type” tumours and that genetic inactivation of MET using siRNA, shifts the epigenotype towards a less aggressive “classical type” tumour.
The above studies suggest that combination treatments involving the targeting of the HGF/c-MET pathway plus a chemotherapeutic agent may be a promising approach to treat PDAC.
Vitamin A
Activated PSC lose cytoplasmic vitamin A (retinol) and transform into a myofibroblast phenotype leading to increased ECM synthesis and fibrosis (18). Han et al. (136) used a tumour microenvironment activated nano system to co-deliver all trans retinoic acid (a metabolite of retinol) and siRNA targeting heat shock protein 47 (HSP 47) to target both activated PSCs and ECM in an orthotopic pancreatic tumour mouse model developed by co-inoculation of luciferase-expressing Panc-1 cells and PSCs. The nanosystem induced quiescence of activated PSCs, inhibited ECM proliferation and improved the efficacy of concomitant gemcitabine treatment.
Vitamin D
Sherman et al. (94) found that activation of the vitamin D receptor (VDR) with calcipotriol in combination with gemcitabine induced quiescence of activated PSCs by inducing Fabp4 (a PSC quiescence marker) and markedly reduced markers of inflammation and fibrosis in KPC mice (a transgenic PDAC model). On the other hand, vitamin D3 is reported to induce differentiation of immature CD34+ myeloid cells into dendritic cells thereby promoting tumour immunity (137). Stemming from the studies reported by Sherman et al. (94) a vitamin D analogue paricalcitol, is currently under clinical trial in combination with gemcitabine, and nab-paclitaxel (NCT03520790).
Epigenetic targeting
Epigenetic modifications play an important role in the growth and progression of PDAC. As these modifications are reversible, targeting them offers new therapeutic opportunities in PDAC. Some studies have shown that targeting epigenetic pathways could help in reprogramming of the tumour microenvironment (138,139) to the detriment of the tumour. DNA methylation, histone modifications, bromodomain and extra-terminal domain (BET) family proteins are the common targets of therapy in PDAC. 5-Azacitidine, a DNA methyl transferase inhibitor is currently being evaluated in a Phase II clinical trial in PDAC resected patients with node positive disease and elevated CA 19-9 (NCT01845805).
Conclusions
In conclusion, there is growing consensus that PSCs have diverse roles in both health and disease and that PSC-cancer cell interactions are central to the progression of PDAC. Many studies are now targeting PSC activation and tumour-stromal interactions for developing better treatments for pancreatic cancer. Better understanding of PSC activation and their interaction with cancer and other stromal elements will aid in mitigating chemoresistance and developing novel therapies in the future for PDAC.
Acknowledgments
Figures 1,2 are reprinted with permission from the publishers Elsevier and Wolters Kluwer Health. Figures 3,4 were created using BioRender.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Min Li) for the series “Science on Pancreatic Cancer” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2019.05.02). The series “Science on Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. All authors report grants from Avner Foundation, grants from National Health and Medical Research Council of Australia, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog 2012;51:53-63. [Crossref] [PubMed]
- Ghiorzo P. Genetic predisposition to pancreatic cancer. World J Gastroenterol 2014;20:10778-89. [Crossref] [PubMed]
- Decker GA, Batheja MJ, Collins JM, et al. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol (N Y) 2010;6:246-54. [PubMed]
- Iodice S, Gandini S, Maisonneuve P, et al. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008;393:535-45. [Crossref] [PubMed]
- Bosetti C, Rosato V, Li D, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol 2014;25:2065-72. [Crossref] [PubMed]
- Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer 2007;120:1993-8. [Crossref] [PubMed]
- Berrington de Gonzalez A, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer 2003;89:519-23. [Crossref] [PubMed]
- Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801-6. [Crossref] [PubMed]
- Morris JP. Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer 2010;10:683-95. [Crossref] [PubMed]
- Cicenas J, Kvederaviciute K, Meskinyte I, et al. KRAS, TP53, CDKN2A, SMAD4, BRCA1, and BRCA2 Mutations in Pancreatic Cancer. Cancers (Basel) 2017; [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol 2015;21:3157-65. [Crossref] [PubMed]
- Gress FG, Hawes RH, Savides TJ, et al. Role of EUS in the preoperative staging of pancreatic cancer: a large single-center experience. Gastrointest Endosc 1999;50:786-91. [Crossref] [PubMed]
- Apte MV, Xu Z, Pothula S, et al. Pancreatic cancer: The microenvironment needs attention too! Pancreatology 2015;15:S32-8. [Crossref] [PubMed]
- Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 2004;29:179-87. [Crossref] [PubMed]
- Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res 2012;18:4266-76. [Crossref] [PubMed]
- Karakhanova S, Link J, Heinrich M, et al. Characterization of myeloid leukocytes and soluble mediators in pancreatic cancer: importance of myeloid-derived suppressor cells. Oncoimmunology 2015;4:e998519 [Crossref] [PubMed]
- Kadera BE, Li L, Toste PA, et al. MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated fibroblasts promotes metastasis. PLoS One 2013;8:e71978 [Crossref] [PubMed]
- Strell C, Norberg KJ, Mezheyeuski A, et al. Stroma-regulated HMGA2 is an independent prognostic marker in PDAC and AAC. Br J Cancer 2017;117:65-77. [Crossref] [PubMed]
- Erkan M, Michalski CW, Rieder S, et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 2008;6:1155-61. [Crossref] [PubMed]
- Fujita H, Ohuchida K, Mizumoto K, et al. alpha-Smooth Muscle Actin Expressing Stroma Promotes an Aggressive Tumor Biology in Pancreatic Ductal Adenocarcinoma. Pancreas 2010;39:1254-62. [Crossref] [PubMed]
- Wang LM, Silva MA, D'Costa Z, et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 2016;7:4183-94. [PubMed]
- Yoneura N, Takano S, Yoshitomi H, et al. Expression of annexin II and stromal tenascin C promotes epithelial to mesenchymal transition and correlates with distant metastasis in pancreatic cancer. Int J Mol Med 2018;42:821-30. [PubMed]
- Zhang D, Li L, Jiang H, et al. Tumor-Stroma IL1beta-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic Cancer. Cancer Res 2018;78:1700-12. [Crossref] [PubMed]
- Mantoni TS, Schendel RR, Rodel F, et al. Stromal SPARC expression and patient survival after chemoradiation for non-resectable pancreatic adenocarcinoma. Cancer Biol Ther 2008;7:1806-15. [Crossref] [PubMed]
- Erkan M, Kleeff J, Gorbachevski A, et al. Periostin creates a tumor-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterology 2007;132:1447-64. [Crossref] [PubMed]
- Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell 2015;28:831-3. [Crossref] [PubMed]
- Bever KM, Sugar EA, Bigelow E, et al. The prognostic value of stroma in pancreatic cancer in patients receiving adjuvant therapy. HPB (Oxford) 2015;17:292-8. [Crossref] [PubMed]
- Yuzawa S, Kano MR, Einama T, et al. PDGFRbeta expression in tumor stroma of pancreatic adenocarcinoma as a reliable prognostic marker. Med Oncol 2012;29:2824-30. [Crossref] [PubMed]
- Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579-96. [PubMed]
- Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 1998;43:128-33. [Crossref] [PubMed]
- Fu Y, Liu S, Zeng S, et al. The critical roles of activated stellate cells-mediated paracrine signaling, metabolism and onco-immunology in pancreatic ductal adenocarcinoma. Mol Cancer 2018;17:62. [Crossref] [PubMed]
- Zhao L, Burt AD. The diffuse stellate cell system. J Mol Histol 2007;38:53-64. [Crossref] [PubMed]
- Wilson JS, Pirola RC, Apte MV. Stars and stripes in pancreatic cancer: role of stellate cells and stroma in cancer progression. Front Physiol 2014;5:52. [Crossref] [PubMed]
- Sparmann G, Kruse ML, Hofmeister-Mielke N, et al. Bone marrow-derived pancreatic stellate cells in rats. Cell Res 2010;20:288-98. [Crossref] [PubMed]
- Ino K, Masuya M, Tawara I, et al. Monocytes infiltrate the pancreas via the MCP-1/CCR2 pathway and differentiate into stellate cells. PLoS One 2014;9:e84889 [Crossref] [PubMed]
- Gonzalez-Villasana V, Rodriguez-Aguayo C, Arumugam T, et al. Bisphosphonates inhibit stellate cell activity and enhance antitumor effects of nanoparticle albumin-bound paclitaxel in pancreatic ductal adenocarcinoma. Mol Cancer Ther 2014;13:2583-94. [Crossref] [PubMed]
- Masamune A, Kikuta K, Watanabe T, et al. Pancreatic stellate cells express Toll-like receptors. J Gastroenterol 2008;43:352-62. [Crossref] [PubMed]
- Shimizu K, Kobayashi M, Tahara J, et al. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology 2005;128:2105-18. [Crossref] [PubMed]
- Morishita K, Shimizu K, Haruta I, et al. Engulfment of gram-positive bacteria by pancreatic stellate cells in pancreatic fibrosis. Pancreas 2010;39:1002-7. [Crossref] [PubMed]
- Sicchieri RD, da Silveira WA, Mandarano LR, et al. ABCG2 is a potential marker of tumor-initiating cells in breast cancer. Tumour Biol 2015;36:9233-43. [Crossref] [PubMed]
- Mato E, Lucas M, Petriz J, et al. Identification of a pancreatic stellate cell population with properties of progenitor cells: new role for stellate cells in the pancreas. Biochem J 2009;421:181-91. [Crossref] [PubMed]
- Berna MJ, Seiz O, Nast JF, et al. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem 2010;285:38905-14. [Crossref] [PubMed]
- Xu Z, Pothula SP, Wilson JS, et al. Pancreatic cancer and its stroma: a conspiracy theory. World J Gastroenterol 2014;20:11216-29. [Crossref] [PubMed]
- Apte MV, Haber PS, Darby SJ, et al. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 1999;44:534-41. [Crossref] [PubMed]
- Pinzani M. Pancreatic stellate cells: new kids become mature. Gut 2006;55:12-4. [Crossref] [PubMed]
- Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 2007;117:50-9. [Crossref] [PubMed]
- Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998;115:421-32. [Crossref] [PubMed]
- Mews P, Phillips P, Fahmy R, et al. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut 2002;50:535-41. [Crossref] [PubMed]
- Casini A, Galli A, Pignalosa P, et al. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol 2000;192:81-9. [Crossref] [PubMed]
- Uden S, Bilton D, Nathan L, et al. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther 1990;4:357-71. [Crossref] [PubMed]
- Apte M, Pirola R, Wilson J. The fibrosis of chronic pancreatitis: new insights into the role of pancreatic stellate cells. Antioxid Redox Signal 2011;15:2711-22. [Crossref] [PubMed]
- Masamune A, Satoh A, Watanabe T, et al. Effects of ethanol and its metabolites on human pancreatic stellate cells. Dig Dis Sci 2010;55:204-11. [Crossref] [PubMed]
- Ben-Harosh Y, Anosov M, Salem H, et al. Pancreatic stellate cell activation is regulated by fatty acids and ER stress. Experimental Cell Research 2017;359:76-85. [Crossref] [PubMed]
- Vonlaufen A, Xu Z, Daniel B, et al. Bacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology 2007;133:1293-303. [Crossref] [PubMed]
- Wang T, Wang QA, Pan GX, et al. ASIC1a involves acidic microenvironment-induced activation and autophagy of pancreatic stellate cells. RSC Advances 2018;8:30950-6. [Crossref]
- Masamune A, Kikuta K, Watanabe T, et al. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am J Physiol Gastrointest Liver Physiol 2008;295:G709-17. [Crossref] [PubMed]
- Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer 2013;108:1-8. [Crossref] [PubMed]
- Nomiyama Y, Tashiro M, Yamaguchi T, et al. High glucose activates rat pancreatic stellate cells through protein kinase C and p38 mitogen-activated protein kinase pathway. Pancreas 2007;34:364-72. [Crossref] [PubMed]
- Kumar K, DeCant BT, Grippo PJ, et al. BET inhibitors block pancreatic stellate cell collagen I production and attenuate fibrosis in vivo. JCI Insight 2017;2:e88032 [Crossref] [PubMed]
- Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature 2010;468:1067-73. [Crossref] [PubMed]
- Lachowski D, Cortes E, Pink D, et al. Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Sci Rep 2017;7:2506. [Crossref] [PubMed]
- Apte MV, Wilson JS, Lugea A, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 2013;144:1210-9. [Crossref] [PubMed]
- Kakizaki Y, Makino N, Tozawa T, et al. Stromal Fibrosis and Expression of Matricellular Proteins Correlate With Histological Grade of Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 2016;45:1145-52. [Crossref] [PubMed]
- Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res 2008;68:2085-93. [Crossref] [PubMed]
- Xu Z, Vonlaufen A, Phillips PA, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol 2010;177:2585-96. [Crossref] [PubMed]
- Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 2008;68:918-26. [Crossref] [PubMed]
- Bachem MG, Schunemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 2005;128:907-21. [Crossref] [PubMed]
- Pandol S, Gukovskaya A, Edderkaoui M, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: role of the stellate cell. J Gastroenterol Hepatol 2012;27:127-34. [Crossref] [PubMed]
- Schneiderhan W, Diaz F, Fundel M, et al. Pancreatic stellate cells are an important source of MMP-2 in human pancreatic cancer and accelerate tumor progression in a murine xenograft model and CAM assay. J Cell Sci 2007;120:512-9. [Crossref] [PubMed]
- Vonlaufen A, Phillips PA, Xu Z, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res 2008;68:7707-10. [Crossref] [PubMed]
- Sousa CM, Biancur DE, Wang X, et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 2016;536:479-83. [Crossref] [PubMed]
- Endo S, Nakata K, Ohuchida K, et al. Autophagy Is Required for Activation of Pancreatic Stellate Cells, Associated With Pancreatic Cancer Progression and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology 2017;152:1492-506.e24. [Crossref] [PubMed]
- Kikuta K, Masamune A, Watanabe T, et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochem Biophys Res Commun 2010;403:380-4. [Crossref] [PubMed]
- Fujita H, Ohuchida K, Mizumoto K, et al. Tumor-stromal interactions with direct cell contacts enhance proliferation of human pancreatic carcinoma cells. Cancer Sci 2009;100:2309-17. [Crossref] [PubMed]
- Hamada S, Masamune A, Takikawa T, et al. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun 2012;421:349-54. [Crossref] [PubMed]
- Sherman MH, Yu RT, Tseng TW, et al. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci U S A 2017;114:1129-34. [Crossref] [PubMed]
- Pothula SP, Xu Z, Goldstein D, et al. Targeting the HGF/c-MET pathway: stromal remodelling in pancreatic cancer. Oncotarget 2017;8:76722-39. [Crossref] [PubMed]
- Neesse A, Wagner M, Ellenrieder V, et al. Pancreatic stellate cells potentiate proinvasive effects of SERPINE2 expression in pancreatic cancer xenograft tumors. Pancreatology 2007;7:380-5. [Crossref] [PubMed]
- Suetsugu A, Snyder CS, Moriwaki H, et al. Imaging the Interaction of Pancreatic Cancer and Stellate Cells in the Tumor Microenvironment during Metastasis. Anticancer Res 2015;35:2545-51. [PubMed]
- Storck H, Hild B, Schimmelpfennig S, et al. Ion channels in control of pancreatic stellate cell migration. Oncotarget 2017;8:769-84. [Crossref] [PubMed]
- Kang N, Gores GJ, Shah VH. Hepatic stellate cells: partners in crime for liver metastases? Hepatology 2011;54:707-13. [Crossref] [PubMed]
- Takikawa T, Masamune A, Yoshida N, et al. Exosomes Derived From Pancreatic Stellate Cells: MicroRNA Signature and Effects on Pancreatic Cancer Cells. Pancreas 2017;46:19-27. [Crossref] [PubMed]
- Masamune A, Kikuta K, Satoh M, et al. Rho kinase inhibitors block activation of pancreatic stellate cells. Br J Pharmacol 2003;140:1292-302. [Crossref] [PubMed]
- Yoshida S, Yokota T, Ujiki M, et al. Pancreatic cancer stimulates pancreatic stellate cell proliferation and TIMP-1 production through the MAP kinase pathway. Biochem Biophys Res Commun 2004;323:1241-5. [Crossref] [PubMed]
- McCarroll JA, Phillips PA, Kumar RK, et al. Pancreatic stellate cell migration: role of the phosphatidylinositol 3-kinase(PI3-kinase) pathway. Biochem Pharmacol 2004;67:1215-25. [Crossref] [PubMed]
- Ohnishi H, Miyata T, Yasuda H, et al. Distinct roles of Smad2-, Smad3-, and ERK-dependent pathways in transforming growth factor-beta1 regulation of pancreatic stellate cellular functions. J Biol Chem 2004;279:8873-8. [Crossref] [PubMed]
- Sugimoto R, Enjoji M, Kohjima M, et al. High glucose stimulates hepatic stellate cells to proliferate and to produce collagen through free radical production and activation of mitogen-activated protein kinase. Liver Int 2005;25:1018-26. [Crossref] [PubMed]
- Rucki AA, Xiao Q, Muth S, et al. Dual Inhibition of Hedgehog and c-Met Pathways for Pancreatic Cancer Treatment. Mol Cancer Ther 2017;16:2399-409. [Crossref] [PubMed]
- Froeling FE, Feig C, Chelala C, et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-beta-catenin signaling to slow tumor progression. Gastroenterology 2011;141:1486-97, 1497.e1-14.
- Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014;159:80-93. [Crossref] [PubMed]
- Zambirinis CP, Levie E, Nguy S, et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J Exp Med 2015;212:2077-94. [Crossref] [PubMed]
- Liu Y, Li F, Gao F, et al. Role of microenvironmental periostin in pancreatic cancer progression. Oncotarget 2016;8:89552-65. [PubMed]
- Li N, Li Y, Li Z, et al. Hypoxia Inducible Factor 1 (HIF-1) Recruits Macrophage to Activate Pancreatic Stellate Cells in Pancreatic Ductal Adenocarcinoma. Int J Mol Sci 2016;17. [PubMed]
- Komar HM, Serpa G, Kerscher C, et al. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci Rep 2017;7:1787. [Crossref] [PubMed]
- Yoshida N, Masamune A, Hamada S, et al. Kindlin-2 in pancreatic stellate cells promotes the progression of pancreatic cancer. Cancer Lett 2017;390:103-14. [Crossref] [PubMed]
- Qian D, Lu Z, Xu Q, et al. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis. Cancer Lett 2017;397:43-51. [Crossref] [PubMed]
- Yan B, Cheng L, Jiang Z, et al. Resveratrol Inhibits ROS-Promoted Activation and Glycolysis of Pancreatic Stellate Cells via Suppression of miR-21. Oxid Med Cell Longev 2018;2018:1346958 [Crossref] [PubMed]
- Garg B, Giri B, Modi S, et al. NFkappaB in Pancreatic Stellate Cells Reduces Infiltration of Tumors by Cytotoxic T Cells and Killing of Cancer Cells, via Up-regulation of CXCL12. Gastroenterology 2018;155:880-91.e8. [Crossref] [PubMed]
- Patel MB, Pothula SP, Xu Z, et al. The role of the hepatocyte growth factor/c-MET pathway in pancreatic stellate cell-endothelial cell interactions: antiangiogenic implications in pancreatic cancer. Carcinogenesis 2014;35:1891-900. [Crossref] [PubMed]
- Erkan M, Reiser-Erkan C, Michalski CW, et al. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia 2009;11:497-508. [Crossref] [PubMed]
- Erkan M, Adler G, Apte MV, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 2012;61:172-8. [Crossref] [PubMed]
- Watanabe K, Hasegawa Y, Yamashita H, et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. The Journal of clinical investigation 2004;114:898-907. [Crossref] [PubMed]
- Delrue L, Blanckaert P, Mertens D, et al. Assessment of tumor vascularization in pancreatic adenocarcinoma using 128-slice perfusion computed tomography imaging. J Comput Assist Tomogr 2011;35:434-8. [Crossref] [PubMed]
- Ding I, Sun JZ, Fenton B, et al. Intratumoral administration of endostatin plasmid inhibits vascular growth and perfusion in MCa-4 murine mammary carcinomas. Cancer Res 2001;61:526-31. [PubMed]
- Strouch MJ, Cheon EC, Salabat MR, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res 2010;16:2257-65. [Crossref] [PubMed]
- Apte M, Pirola RC, Wilson JS. Pancreatic stellate cell: physiologic role, role in fibrosis and cancer. Curr Opin Gastroenterol 2015;31:416-23. [Crossref] [PubMed]
- Han L, Ma J, Duan W, et al. Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway. Oncotarget 2016;7:18146-58. [PubMed]
- Li X, Wang Z, Ma Q, et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin Cancer Res 2014;20:4326-38. [Crossref] [PubMed]
- Kikuta K, Masamune A, Hamada S, et al. Pancreatic stellate cells reduce insulin expression and induce apoptosis in pancreatic beta-cells. Biochem Biophys Res Commun 2013;433:292-7. [Crossref] [PubMed]
- Schönauer R, Els-Heindl S, Beck-Sickinger AG. Adrenomedullin - new perspectives of a potent peptide hormone. J Pept Sci 2017;23:472-85. [Crossref] [PubMed]
- Drifka CR, Loeffler AG, Esquibel CR, et al. Human pancreatic stellate cells modulate 3D collagen alignment to promote the migration of pancreatic ductal adenocarcinoma cells. Biomed Microdevices 2016;18:105. [Crossref] [PubMed]
- Sada M, Ohuchida K, Horioka K, et al. Hypoxic stellate cells of pancreatic cancer stroma regulate extracellular matrix fiber organization and cancer cell motility. Cancer Lett 2016;372:210-8. [Crossref] [PubMed]
- Horioka K, Ohuchida K, Sada M, et al. Suppression of CD51 in pancreatic stellate cells inhibits tumor growth by reducing stroma and altering tumor-stromal interaction in pancreatic cancer. Int J Oncol 2016;48:1499-508. [Crossref] [PubMed]
- Kang Y, Roife D, Lee Y, et al. Transforming Growth Factor-beta Limits Secretion of Lumican by Activated Stellate Cells within Primary Pancreatic Adenocarcinoma Tumors. Clin Cancer Res 2016;22:4934-46. [Crossref] [PubMed]
- Liu Y, Li F, Gao F, et al. Periostin promotes the chemotherapy resistance to gemcitabine in pancreatic cancer. Tumour Biol 2016;37:15283-91. [Crossref] [PubMed]
- Phillips P. Pancreatic stellate cells and fibrosis. In: Grippo PJ, Munshi HG, editors. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India), 2012.
- McCarroll JA, Naim S, Sharbeen G, et al. Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front Physiol 2014;5:141. [Crossref] [PubMed]
- Pothula SP, Xu Z, Goldstein D, et al. Hepatocyte growth factor inhibition: a novel therapeutic approach in pancreatic cancer. Br J Cancer 2016;114:269-80. [Crossref] [PubMed]
- Singh S, Srivastava SK, Bhardwaj A, et al. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer 2010;103:1671-9. [Crossref] [PubMed]
- Cao F, Li J, Sun H, et al. HES 1 is essential for chemoresistance induced by stellate cells and is associated with poor prognosis in pancreatic cancer. Oncol Rep 2015;33:1883-9. [Crossref] [PubMed]
- Hessmann E, Patzak MS, Klein L, et al. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut 2018;67:497-507. [Crossref] [PubMed]
- Mantoni TS, Lunardi S, Al-Assar O, et al. Pancreatic stellate cells radioprotect pancreatic cancer cells through beta1-integrin signaling. Cancer Res 2011;71:3453-8. [Crossref] [PubMed]
- Al-Assar O, Demiciorglu F, Lunardi S, et al. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother Oncol 2014;111:243-51. [Crossref] [PubMed]
- Walter K, Omura N, Hong SM, et al. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res 2010;16:1781-9. [Crossref] [PubMed]
- Khan S, Ebeling MC, Chauhan N, et al. Ormeloxifene suppresses desmoplasia and enhances sensitivity of gemcitabine in pancreatic cancer. Cancer Res 2015;75:2292-304. [Crossref] [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Kim EJ, Sahai V, Abel EV, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res 2014;20:5937-45. [Crossref] [PubMed]
- Li C, Wu JJ, Hynes M, et al. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology 2011;141:2218-27.e5. [Crossref] [PubMed]
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313-23. [Crossref] [PubMed]
- Quint K, Tonigold M, Di Fazio P, et al. Pancreatic cancer cells surviving gemcitabine treatment express markers of stem cell differentiation and epithelial-mesenchymal transition. Int J Oncol 2012;41:2093-102. [Crossref] [PubMed]
- Lomberk G, Blum Y, Nicolle R, et al. Distinct epigenetic landscapes underlie the pathobiology of pancreatic cancer subtypes. Nat Commun 2018;9:1978. [Crossref] [PubMed]
- Han X, Li Y, Xu Y, et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat Commun 2018;9:3390. [Crossref] [PubMed]
- Kulbersh JS, Day TA, Gillespie MB, et al. 1alpha,25-Dihydroxyvitamin D(3) to skew intratumoral levels of immune inhibitory CD34(+) progenitor cells into dendritic cells. Otolaryngol Head Neck Surg 2009;140:235-40. [Crossref] [PubMed]
- Nguyen AH, Elliott IA, Wu N, et al. Histone deacetylase inhibitors provoke a tumor supportive phenotype in pancreatic cancer associated fibroblasts. Oncotarget 2017;8:19074-88. [Crossref] [PubMed]
- Yamamoto K, Tateishi K, Kudo Y, et al. Stromal remodeling by the BET bromodomain inhibitor JQ1 suppresses the progression of human pancreatic cancer. Oncotarget 2016;7:61469-84. [Crossref] [PubMed]
Cite this article as: Mekapogu AR, Pothula SP, Pirola RC, Wilson JS, Apte MV. Multifunctional role of pancreatic stellate cells in pancreatic cancer. Ann Pancreat Cancer 2019;2:10.


