
Preclinical mouse models for immunotherapeutic and non-immunotherapeutic drug development for pancreatic ductal adenocarcinoma
Molecular pathogenesis of pancreatic ductal adenocarcinoma (PDAC)
PDAC is the third leading cause of all cancer-related deaths. It has a 5-year survival rate of only 9% in the United States (1). The low survival rate is mainly due to early metastasis and limited therapeutic options. Only 10–20% of patients are diagnosed with a surgically resectable tumor due to delay in diagnosis with a 5-year survival rate of 37%, whereas 53% of patients are diagnosed at a metastatic stage with a 5-year survival rate at 3% (1,2). Common therapeutic options for PDAC patients include surgery, radiation therapy, and chemotherapy, which aim at relieving symptoms and/or extending survival. Surgery is the only possible cure, but it is only suitable for less than 20% of the patients (1,3). Hence, it is of vital importance to develop new diagnostic tools and therapeutic strategies, which rely heavily on the use of proper preclinical models.
In the past couple of decades, the mechanism for the pathogenesis of PDAC has been extensively studied. It is a common belief that mutations in KRAS, CDKN2A, TP53, BRCA2 and SMAD4 are important for PDAC tumorigenesis (3). The oncogenic mutation in KRAS is considered the first genetic change to initiate pancreatic intraepithelial neoplasia (PanIN) lesions and are found in nearly 90% of invasive PDAC tumors in patients (4). Mutational activation of KRAS results in induction of cell proliferation, cell survival, invasion, and stimulation of other oncogenic signaling pathways (5). Different KRAS mutations can occur within the same PanIN lesion and PDAC tumor, further supporting its role in PDAC progression. The loss of the tumor suppressor gene CDKN2A, encoding for p16 (INK4A), is another common mutation found in 90% of the PDAC tumors (6). The gene CDKN2A encodes cyclin-dependent kinase (CDK) inhibitor which inhibits cell cycle progression. Hence, the loss of CDKN2A function accelerates PDAC progression (7). Loss of tumor suppressor gene TP53 function via missense alteration of the DNA-binding domain occurs in more than 70% of PDAC patients, contributing to genomic instability and telomere dysfunction during cancer progression (8,9). Genetic alterations resulting in loss of SMAD4 function occur in more than 50% of PDAC patients. The SMAD4 protein is critical for transforming growth factor beta (TGF-β) signaling. Loss of SMAD4 function abrogates the SMAD4-dependent TGF-β pathway, promoting cancer cell growth (10). Decrease expression of PTEN has also been found in 70% of PDAC patients, suggesting its role as a major tumor suppressor in PDAC (11). The reduced level of PTEN is associated with an enhanced PI3K/Akt signaling, promoting PDAC metastasis (12).
Due to the importance of these common genetic alterations in PDAC pathogenesis, transgenic mouse models of PDAC have been developed to recapitulate these genetic alterations and study their specific role(s) in the molecular pathogenesis of PDAC.
In addition to genetic mutations, tumor-stroma interactions within the heterotypic microenvironment also greatly contribute to the pathogenesis of PDAC (3). The majority of PDAC tumors are predominately composed of fibroblasts, endothelial cells, extracellular matrix (ECM), hematopoietic cells, and myeloid cells (13). Deposition of ECM components and proliferation of stromal fibroblasts are often found within the tumor microenvironment (TME), contributing to the complexity of the TME in PDAC. Pancreatic stellate cells (PSCs) are another major component within the TME and once activated, they transition to myofibroblasts to secret ECM proteins (14,15). Fibroblasts can also negatively impact the immune cell infiltration in the TME by secreting CXCL12 to prevent the entering of CXCR4+ T cells into the TME (16). Secreting a panel of chemokines with heterogeneous functionalities including CCL5, CCL2, CCL17, IL-1, IL-4, IL-13, and IL-23, fibroblasts can also hinder macrophages and T cell functions (15-17). A strong immunosuppressive microenvironment is a renowned characteristic of PDAC, achieved by a high number of myeloid cells including myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs) (13). Coupled with the relative absence of T cells within the TME, the poor response rates of chemotherapy, targeted therapy, and immunotherapy in treating PDAC is attributed to the desmoplastic immunosuppressive TME. Therefore, it is essential for a preclinical animal model to recapitulate the TME of human PDAC.
Thus, an ideal preclinical model of PDAC needs to represent both molecular pathogenesis and the TME of human PDAC. In this review, we discuss the advantages and disadvantages of key animal models that are currently used in preclinical research (Tables 1,2).
Table 1
| Characteristics | Relative levels |
|---|---|
| Skills/facility/maintenance required | Cell lines < patient-derived mouse models < syngeneic mouse models = humanized mouse models < GEMMs |
| Easy to manipulate genetic background | GEMMs < syngeneic mouse models = patient-derived mouse models = humanized mouse models < cell lines |
| Time consumption | Cell lines < syngeneic mouse models = GEMMS = patient- derived models = humanized mouse models |
| Clinical relevance | Cell lines < syngeneic models < GEMMS < patient-derived models = humanized mouse models |
GEMMS, genetically engineered mouse models.
Table 2
| Early stage PDAC | Immune system | Tumor microenvironment | Metastasis | Large-scale and/or high- throughput drug screening | |
|---|---|---|---|---|---|
| Cell lines (mouse-derived & patient derived) | No | Limited | No | No | Yes |
| Patient derived xenograft models—SQ | No | Limited | No | No | No |
| Patient derived xenograft models—orthotopic | No | Yes | Yes | Limited | No |
| Humanized mouse | No | Yes | Yes | Yes | No |
| Genetically engineered mouse model | Yes | Yes | Yes | Yes | No |
| Syngeneic mouse model—subcutaneous | No | Yes | No | No | No |
| Syngeneic mouse model—orthotopic | No | Yes | Yes | Limited | No |
| Syngeneic mouse model—hemispleen | No | Yes | Yes | Yes | No |
PDAC, pancreatic ductal adenocarcinoma.
Patient-derived xenograft models
Patient-derived PDAC models were one of the first preclinical models used for PDAC research. As early as 1963, researchers had utilized human PDAC cell lines derived from primary pancreatic tumors to characterize and test anti-cancer drugs (18). These stable immortal cell lines are relatively homogenous, easy to use, and cost-effective. Through proteomic and transcriptomic approaches, studies have identified key characteristics of PDAC cell lines including mutations in KRAS, p53, and SMAD4. Transcriptome analysis of PDAC cell lines has also revealed a list of oncogenic miRNAs regulating tumor promoting genes like TP53, Bcl2, Rac1, and CD40 (19-22). Despite the convenience of patient derived cell lines, they are less than optimal for PDAC studies. Maintained in culture, highly mutative cancer cell lines may accumulate genetic changes over multiple passages, and thus may generate new characteristics to impact the reproducibility of related experiments. In addition, different PDAC cell lines can cause differences in research outcomes, failing bench-to-bedside transition (23,24). The limited variety of human PDAC cell lines can only represent a limited patient population (25). Substantial differences in protein expressions exist between cell lines and tumors in patients. In addition, the PDAC cell line maintained as monolayer culture may be selected for subpopulations with additional mutations that result in growth advantages (26,27). Therefore, direct patient-derived tumor tissue specimens are utilized to minimize the above-described disadvantages of the established monolayer cultured cell lines. They are subcutaneously implanted (under the skin) in the immunocompromised mice and passaged from one mouse to another. Pieces of dissected tumors can be cryopreserved for long-term storage. However, the success rate of direct tumor tissue implantation is dependent on the aggressiveness of the cell lines or the resected tissues. Thus, the growth of implanted tumors is correlated with poor prognosis and more aggressive tumors in patients (4,6). However, using direct tumor tissue implantation also provides the potential for personalized medicines. By either injecting tumor cells or tumor pieces derived from tumor excision or biopsy, mice with those tumors harbor intratumoral heterogeneities as the patient (28). Researchers and physicians can then better predict the outcome of a treatment by testing drugs and therapeutic methods on those mice. Yet, generating a patient-derived model from primary tissues can take up to eight months, making it challenging for routine diagnostic use in a clinical setting given the short survival time of PDAC patients.
Commonly used xenograft models include cell line-derived xenograft (CDX) or xenograft (PDX) model by introducing human PDAC cell lines or PDAC tumor tissues into immunocompromised mice respectively (29-31). The discoveries of T cell deficient nude athymic mice, as well as B and T cell deficient severe combined immunodeficient (SCID) mice, allowed researchers to overcome the species barrier to develop xenograft models using human specimens (32,33). Stable human pancreatic cell lines or resected tumor tissues can be injected or transplanted into a mouse subcutaneously (34). Such an approach is often favored for non-immunotherapeutic drug screening as it is relatively cost-effective and convenient (35). The implanted tumor is easy to visualize and easy to measure for determining drug efficacy. Depending on the aggressiveness and invasiveness of the cell lines, tumors can be palpable within 2–6 weeks (36). However, the biological relevance of subcutaneous models is limited as PDAC patients often develop metastasis, yet subcutaneous murine models often do not. In addition, drug delivery and tissue penetration in human patients would not be recapitulated in the subcutaneous models of PDXs and CDXs.
Compared to subcutaneous models, orthotopic xenograph models, implanting PDAC cells or resected tissues into the pancreas of nude mice or SCID mice, allow a better resemblance of human PDAC (37,38). Although the procedure requires higher surgical techniques and is more costly, it has a higher predictive value to generate more biologically relevant data (29). Orthotopic models often show stable growth kinetics, molecular diversities, and measurable metastasis, allowing for better identification of tumor genotypes and morphologies, as well as non-immunotherapeutic drug testing (39,40). However, continuous monitoring of tumor growth is challenging in orthotopic. The current common method is through ultrasonography. Nude and SCID murine models are more susceptible to infections and other health problems with their compromised immune system, potentially obstructing experiments. In addition, during the generation of PDX, a subpopulation of tumor cells with stronger proliferative advantages is likely to outgrow the others, resulting in an inevitable selection of more aggressive cancers in xenograft models, and therefore limiting the researchable targets and cancer genotypes (41-43). As immunocompromised murine models lack heterogeneous stroma and an intact immune system, they are not ideal for the development of drugs targeting TME and the immune system.
Humanized mouse model
To compensate for the immunodeficiency of SCID or nude mice while maintaining the clinical relevance of using patient-derived tumor cells and tissues, humanized mice bearing mutations in the IL2 receptor common gamma chain (IL2rgnull) in the non-obese diabetic (NOD)/SCID background were developed (44-46). With less NK cell activity from NOD background and the severely impaired B and T cell functions from SCID background, these mice support engraftment with human tissue, peripheral-blood mononuclear cells (PBMCs) and hematopoietic stem cells (HSCs), enabling the modeling of human immunity in immunocompromised mice (47,48). PBMCs allow the introduction of mature human leukocytes, especially activated T cells, whereas HSCs can potentially introduce all human hematopoietic lineages (49). Three commonly used humanized mice strains are: NOD.Cg-PrkdcscidIl2rgtm1Wjl (NSG), C;129S4-Rag2tm1FlvIl2rgtm1Flv (BRG), and NODShi.Cg-PrkdcscidIl2rgtm1Sug (NOG) (47,49). While NOG mice have truncated IL2 receptor common gamma chain, NSG and BRG mice have complete null allele of the gamma chain.
Under this unique genetic background, patient-derived PDAC tissues or cell lines can be transplanted into these mice while potentially maintaining the tumor and TME heterogeneities. In general, compared to patient-derived cell lines, PDXs are better for therapeutic screening with higher correlation of clinical efficacy (49). Recently, a ‘AVATAR’ approach had also been taken to use humanized mice for personalized medicine to test for the efficacy of treatments (50).
Engraftments of human immune components enable characterization of interactions between the tumor and the immune systems, as well as providing valuable insights for both cell-based and antibody-based immunotherapy development (51). For instance, genetically modified T cells with chimeric antigen receptors (CAR-T cells) have been introduced into NSG mice to investigate the anti-tumor potentials of immunotherapies (52,53). However, CAR-T therapy showed strong adverse events in patients but not in humanized mice, possibly due to the lack of human targets in normal mouse tissues. Another popular approach using humanized models for PDAC cellular immunotherapy is called adoptive NK cell therapy aiming at stimulating the anti-tumor activity of NK and NKT cells (54). Humanized positive and negative immunological regulators and ligands of interests have also been knock-in to immunocompromised mice, including PD-L1, CD47, BTLA, OX40, etc., providing valuable research tools for studies of clinical candidates, especially combination therapies targeting immune-oncology checkpoints. Humanized mice also allow the studies of human antibody-dependent cellular cytotoxicity (ADCC).
Although humanized mouse models allow investigations of novel immunotherapies, these animals do not harbor the full human immune system. The remnant mouse innate immunity in humanized mice results in limited lymph node development, HLA incompatibility between engrafted human immune system and implanted PDX, and an inability to mimic human immune cell trafficking, all of which are major shortcomings in the currently used humanized mouse models (55). Pancreatic cancers are traditionally classified as non-immunogenic (“cold”) tumors due to its lack of T cell infiltrations. Such property of human PDAC partially explains the negative outcome of immune checkpoint inhibitors in clinical settings (56). Yet, in current PDAC research, tumor implantations can cause T cell infiltrations into the tumors due to histoincompatibility, subsequently changing these non-immunogenic “cold” tumors into artificially immunogenic “hot” tumors. Therefore, introducing human tumors into immunocompromised mice can still be recognized as foreign substances, causing T cell infiltration in the TME and leading to false results in immunotherapeutic studies (56,57).
Genetically engineered mouse model (GEMM)
Although immunocompromised mouse models using human cells and tissues allow good representation of human disease, immunocompetent mouse models of PDACs are still the mainstream of preclinical mouse models. KRAS mutations occur in more than 90% of PDAC patients (58). In addition, endogenously expressing KrasG12D allows the initiation of PanIN, which can spontaneously progress into aggressive and metastatic diseases. Taken the abundance and pathogenic significance of KRAS mutation, researchers had started generating genetically engineered mouse models (GEMMs) harboring KRAS mutations. Although KRAS mutation alone is not sufficient to induce PDAC, in combination with other common PDAC mutations like INK4A, TP53, SMAD4, and TGF-β, various GEMMs had been developed on the base of KrasG12D mutations (59,60) (Table 3).
Table 3
| GEMMs | Genetic mutation(s) | Time of mutant expression | Average time of tumor formation | Severity of PDAC development | Median survival | Reference |
|---|---|---|---|---|---|---|
| KP Mice with Pdx1- Cre | LSL-KrasG12D; Pdx1-Cre | E8.5 | 6.25 months | From PanINs to aggressive and invasive PDAC in an age- dependent manner | 1.5 years | (58) |
| KP Mice with P48+/Cre | LSL-KrasG12D; P48+/Cre | E9.5 | 8.25 months | From PanINs to aggressive and invasive PDAC in an age- dependent manner | 1.5 years | (58) |
| KPC Mice | LSL-KrasG12D; LSL-Trp53R173H; Pdx1-Cre | E8.5 | 2 to 3 months | From PanINs to aggressive and invasive PDAC in an age- dependent manner | 5 months | (61) |
| KPC-Brca Mice | LSL-KrasG12D; LSL-Trp53R270H; Pdx1-Cre; Brca2Tr | E8.5 | 1.5 months | Aggressive and invasive PDAC in an age- dependent manner | 2.8 months | (62) |
| KC-Ink4a/Arf Mice | LSL-KrasG12D; Pdx1-Cre; Ink4a/Arflox/lox | E8.5 | 1.25 months | Primarily locally invasive tumor | 2 months | (60) |
| KC-Smad4 Mice | LSL-KrasG12D; Pdx1-Cre; Smad4lox/lox | E8.5 | 7 to 12 weeks | Moderate PDAC | 8 to 24 weeks | (63) |
| KC-Tgfb2 Mice | LSL- KrasG12D/+; Ptf1acre/+; Tgfbr2flox/+ | E9.5 | 6 to 7 weeks | Aggressive an Invasive PDAC | 8.4 weeks | (64) |
| Inducible KrasG12V Mice | Kras+/LSLG12Vgeo; Elas-tTA/tetO-Cre | Inducible | Inducible | Dependent on Kras Mutant Induction | Dependent on Kras mutant expression | (65) |
| KPP Mice | LSL-KrasG12D/+; Ptf1aER-Cre/+; Ptenf/f | E9.5 | Initiated with tamoxifen between 24 and 28 days | Moderate PDAC, but progressive cachexic phenotype | 3.5 months | (66) |
GEMMS, genetically engineered mouse models; PDAC, pancreatic ductal adenocarcinoma.
Previous studies had identified a series of important transcription factors during the development of the normal pancreas including early developmental homeodomain-containing transcription factors MNX1, NKX6-1, and PDX1, as well as basic helix-loop-helix transcription factor p48 (67,68). In mice, dorsal and ventral prepancreatic regions are formed independently at around embryonic day E8.5, followed immediately by the appearance of epithelial buds at E9.0 (69). Under the expressions of sonic hedgehog (SHH), retinoic acid (RA), fibroblast growth factor (FGF), and bone morphogenetic protein (BMP), the dorsal and ventral prepancreatic regions are specified (70). To further establish the pancreatic identity during the embryonic stage,PDX1 is expressed to induce the morphogenesis of pancreatic epithelium and pancreatic endocrine cell differentiation (71). The importance of PDX1 during pancreatic development was shown in PDX1-deficient mice as they develop only the pancreas buds, but not functional pancreas (71,72). P48, bound to transcription factor PTF1, is essential for exocrine cell differentiation and proliferation (73). The inactivation of PTF1-p48 changes the fate of pancreatic progenitor cells to duodenal epithelium cells (74). Due to the importance of PDX1, LSL-KrasG12D; Pdx1-Cre mice have subsequently been engineered and used for pancreatic cancer studies. In short, a Kras mutation commonly found in PDAC patients was generated on exon 1 by changing a G to a D, V or R at codon 12 (61). A vector containing the genetic material that inhibits transcription generated with two functional LoxP sites flanking the genetic elements was then inserted into the upstream of the mouse genomic Kras locus (58). The LSL-KrasG12D mice were then interbreed with Pdx1-Cre mice, where the Cre recombinase was expressed only in pancreas (Figure 1A). Through a series of excision-recombination events, the LSL-KrasG12D; Pdx1-Cre animals only express mutant KRAS in the pancreas. Such endogenous expression of KRAS mutant initiates PanINs when the animals are as young as two weeks old. As the animals age, higher-grade of PanINs occur and with higher frequencies.

Similarly, a more robust transgenic mouse model was generated by introducing LSL-Trp53R172H into LSL-KrasG12D animals and then interbreed LSL-KrasG12D; LSL-Trp53R172H; Pdx1-Cre mice with Pdx1-Cre mice (75) (Figure 1B). The resulting LSL- KrasG12D; LSL-Trp53R173H; Pdx1-Cre (KPC) triple mutant animals develop spontaneous PDAC with cachexia, abdominal distension, bowel and biliary obstruction, corresponding to the typical clinical findings in PDAC patients. PDAC progression in KPC mice also closely resemble the human disease as they develop PanIN by the age of 8 to 10 weeks, and invasive tumors by the age of 16 weeks (63). As the disease progresses, the tumor will metastasize to lung, liver, diaphragm, and adrenals in these animals, mirroring human PDAC metastasis. KC-Brca mice have also been engineered harboring mutations in tumor suppressor genes Brca2 and Kras, showing that BRCA2 mutation promotes KRAS-driven pancreas carcinogenesis (62). BRCA2 mutation has also been introduced into KPC mice, providing a more clinically relevant model for PDAC research (62,76). The KC-Brca mice and KPCB mice develop pancreatic tumors in two to three weeks but with a shorter survival of approximately 4 to 5 weeks. Similarly, since the inactivation of G1 cyclin-kinase inhibitor p16INK4A is found in majority of PDAC patients, mice with loss-of-function p16 in combination of KRAS mutation develop highly invasive tumor and died by the age of 11 weeks (60). SMAD4 mutation has also been introduced into KC mice. Mice with SMAD4 mutations and KRAS mutation exhibit early rapid development of intraductal papillary mucinous neoplasia (IPMN), yet failed to develop aggressive pancreatic malignancies (63). Mice with TGF-𝛽 knockout and KrasG12D mutant driven by Ptf1a-Cre-LoxP system were also generated as 55% of PDAC patients have TGF-𝛽 mutation. TGF-𝛽 knockout promotes KRAS-driven tumor by transforming PanINs into PDAC tumors. KC-Tgfb2 mice develops tumors at the age of 6–7 weeks with a medium survival of 8.4 weeks (64). Recently, Talbert et al. has developed a LSL-KrasG12D/+; Ptf1aER-Cre/+; Ptenf/f (KPP) model to recapitulate cancer-induced cachexia (66). Cachexia is a common cancer-induced syndrome characterized by skeletal muscle loss, relating to increased morbidity and mortality (77). PDAC patients often meet the criteria of cachexia upon diagnosis. However, there is no good treatment targeting cancer-induced cachexia, partially due to the lack of an appropriate animal model. Previously, the KPC model was often used for cachexia-associated research as they exhibit cachexia syndromes. However, Talbert et al. has demonstrated that the cachexic phenotypes of KPC are different from the cachexic phenotypes in PDAC patients where their novel KPP model can better represent the progressive wasting phenotype in human PDAC.
As Pdx1- or Ptf1a/p48-driven Cre-LoxP systems allow expression of KrasG12D mutant starting in early pancreatic development and in all epithelial progenitor cells, it is hard to pinpoint the cell-of-origin of PanINs and PDAC tumors (78). In addition, human PanINs and PDAC tumor rarely initiate during pancreatic development. To address this issue, temporal KRAS mutant mice can be generated by crossing mice with conditional endogenous KrasG12V oncogenes in acinar pancreatic cells with bitransgenic Elas-tTA/tetO-Cre mice that can express an Elastase promoter controlling Cre recombinase in a tet-off system (65). Using X-gal staining, 𝛽-galactosidase activity served as a marker for the expression of KRAS mutant under stimulation, proving the selective expression of KrasG12V in acinar and centroacinar pancreatic cells, inducing PanINs and invasive PDAC. An inducible system can also be applied to studies of other oncogenes and tumor suppressor genes. For instance, in combination of a Cre-LoxP system and a Flp-FRT system, YAP expression can be switched off in the background of spontaneous KRAS mutated pancreatic tumors in immunocompetent mice (79). By incorporating fluorescence tags to the YAP protein, the expression pattern of YAP and its effect in PDAC development was revealed. In addition, in the settings of understanding epithelial-to-mesenchymal transitions of pancreatic cells, a yellow fluorescence protein (YFP) protein can be introduced into KPC mice by crossing KPC mice with Pdx1-Cre; RosaYFP mice (80). Using this GEMM, researchers were able to trace YFP tagged mutated pancreatic cells migration even before signs of PDAC tumors. GEMMs provide an opportunity to study not only the establishment, but also the progression of tumors. They reveal valuable information for cancer metabolism and cancer prevention, especially on delaying the precursor lesion progression and preventing both local and metastatic diseases.
As transgenic mouse models recapitulate the tumorigenesis of PDAC, they are useful tools for novel biomarker discoveries in early stages like PanIN as well as in the metastatic stage. Using a proteomics approach, a recent study found an enrichment of a cell surface proteoglycan, glypican-1 (GPC-1) in cancer cell-derived exosomes using the KPC model (81). Levels of GPC+ circulating exosomes correlate with tumor burden and survival in both patients and KPC mice, providing a potential non-invasive diagnostic biomarker for early PDAC detection (82). Other molecules important for PDAC development and metastasis including USP9X (83), Annexin A2 (84), and cytokine tissue inhibitor of matrix metalloproteinases 1 (TIMP1) (85) were also characterized using GEMMs. In addition to the biomarker discoveries, these mouse models also allow developments of novel therapeutic strategies. Preclinically evaluated in KPC mice, pegylated recombinant human hyaluronidase (PEGPH20) was developed to degrade hyaluronic acid (HA) in the ECM, enhancing the drug delivery efficiency (86). Characterization and preclinical study of IPI-926 in KPC mice, an inhibitor of hedgehog signaling pathways in KPC, also lead to the development of a series of hedgehog signaling protein inhibitors and combinational therapeutic strategies (87-89). Unfortunately, human clinical trials of IPI-926 plus gemcitabine or FOLFIRINOX were terminated early due to severe detrimental effects (90). Focal adhesion kinase (FAK) inhibitors had previously found to reduce tumor progression and are recently studied in KPC mouse models in combination with immune checkpoint inhibitors (91).
GEMMs are also largely used for PDAC immunobiology studies as well as PDAC immunotherapy developments. Since PDAC lesion arises spontaneously in the GEMMs, especially the KPC mouse model, it reproduces the leukocyte complexity and the immune cell infiltration in the TME as observed in human PDAC patients (16,63,92,93). For instance, strong infiltration of F4/80+ macrophages and low levels of effector T cells were found in the primary lesion in both KPC mice and PDAC patients (64,94). Preclinical trials or “co-clinical” trials of immunotherapies have been utilizing these GEMMs. Simultaneously conducting a human trial and mouse studies revealed that a CD40 agonist can recruit circulating monocytes to exhibit anti-tumor and anti-fibrotic effects, causing tumor progression in both KPC mice and humans (95,96). KPC models have also enabled studies to limit T cell infiltration into the tumor tissue including CXCL12 leading to a clinical trial of a CXCR4 inhibitor therapy in combination with anti-PD-L1 antibody (16).
In short, GEMMs represents the whole tumorigenesis of PDAC in a mammalian system, allowing researches for cancer preventions and metastasis prevention. While GEMMs are extensively used in current PDAC research, they are expensive and time consuming. In addition, it is hard to isolate and characterize the tumor from the animal due to the lack of neoplastic cellularity. The most common method for tumor growth measurement and monitoring using ultrasonography is time consuming and labor intensive (64,97). Due to the challenges of monitoring tumor sizes throughout the experiments, the outcomes of therapeutic testing using GEMMs are often evaluated by survivals. In addition, individual difference of tumorigenesis between mice often complicate the results of experiments. Intrinsic differences between rodent and human proteins also diminish the predictive values of GEMMs in preclinical research.
Syngeneic mouse models
As the therapeutic role of the immune system has been increasingly recognized, it is pivotal to have a mouse model with a competent immune system. Syngeneic mouse models, developed by introducing genetically similar or identical, or immunologically compatible tumor cells or tumor tissues into immunocompetent mice orthotopically or subcutaneously, are good models for such studies. One of the earliest murine PDAC cell lines, Panc02, was established from chemically induced PDAC mouse models in 1984 (98). However, the genotype and phenotype of Panc02 fail to recapitulate the human PDAC disease as it does not harbor KRAS, p53, and PDX1 mutations (99). A good alternative is the KPC cell lines established from genetically engineered KPC mouse for their representative genetic mutations (100). However, recent reports showed that KPC cell lines are poorly immunogenic due to their similar growth rates in immunodeficient mice and in immunocompetent mice (101). This characteristic of the KPC cell line makes it an ideal model to recapitulate the “cold” TME of human PDAC. The orthotopic model can also more faithfully recapitulate the TME of human PDAC than the subcutaneous model. Another advantage of the orthotopic implantation model is to allow the control of the size of the implanted tumor pieces, simply by carefully cutting the tumor into pieces using a ruler, allowing for the comparison among multiple treatment groups and different combination therapies.
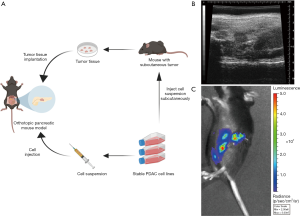
Syngeneic mouse models can be established in immunocompetent mice orthotopically into the pancreas, or subcutaneously under the skin. Transplantation of mouse-derived tumor tissues or injection of mouse-derived tumor cell lines allow the development of tumor and subsequently the intervention on the tumor under a competent immune system. Since the subcutaneously implanted tumor is not growing in its native organs, the biological relevance of subcutaneous models is limited compared to the orthotopic models. Syngeneic orthotopic models are also better at representing the cell-to-immune system interactions. However, recent reports showed that the tumor infiltration B cells in syngeneic orthotopic models are significantly less than in genetically engineered KPC mice, indicating that two models are still different and therefore should be used together for immunotherapeutic development (102). For instance, using a combination of GEMMs and orthotopic models, Foley et al. revealed the metastatic mechanism of PDAC characterized by SEMA3D autocrine signaling (103). Similarly, the preclinical efficacy of GM-SCF-secreting allogenic whole pancreatic tumor cell vaccine (GVAX) targeting Annexin A2 (ANXA2) was evaluated (104). It should be noted that tumor measurement in both GEMMs and orthotopic models can be challenging and time-consuming. Nevertheless, some groups have developed expertise in using small animal ultrasonography to measure the tumors growing on the internal organs. In addition, luciferase can be genetically engineered into the cancer cell lines, enabling a more convenient tumor imaging measuring the intensity of bioluminescence (Figure 2).
Intravenous, intraperitoneal, and intrasplenic administration of mouse-derived cell lines also provides the capabilities to study lung metastasis, peritoneal and lymph node metastasis, and hepatic metastasis respectively (64,105,106). Since the tumor is established at secondary locations instead of its native organs, these models better represent the formation and characteristics of metastasized tumors after surgical resection. As most cases of PDAC were diagnosed at late stage and complicated by distal metastasis, preclinical models of metastases are particularly valuable for developing therapeutics that can target both primary tumors and metastases. Subcutaneous models rarely metastasize. The timing and progression of metastasis in orthotopic models and GEMMs largely vary and thus are not feasible for drug development purposes. Therefore, intravenous, intraperitoneal, and intrasplenic models are commonly used for lung, peritoneal and lymph node, and liver metastasis studies respectively.
In 2014, Soares et al. have developed an intrasplenic model to better study liver metastasis without the presence of primary pancreatic tumor. In short, the hemisplenectomy procedure is achieved by dividing the spleen in half followed by the injection of Panc02 or KPC tumor cells into a hemispleen with splenic blood vessels connecting to the liver (Figure 3) (105). The tumor cells will then travel to and seed in the liver through the splenic blood vessel. To prevent the peritoneal drop metastasis, the injected half of the spleen is removed, while the remaining half of the spleen continues to perform immunological and biological functions (64).
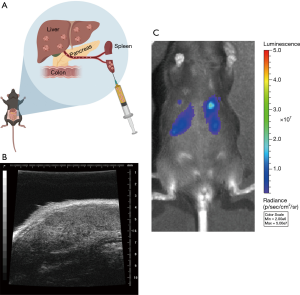
This model was first developed using Panc02 cells, a highly aggressive and tumorigenic, chemically induced mouse cell line in C57BL/6 mice (98). Therefore, despite the long history and widespread usage of Panc02 cell line, Panc02 cells lack clinical significance as it does not harbor representative mutations as in the human disease (100). Compared to Panc02 cell lines, KPC cell lines and other KC-derived cell lines are better representations of the human PDAC disease as they share more genetic mutations. If left untreated, mice with hemispleen tumor would die within a short period of time, typically 30–60 days. Yet, using Panc02 cell lines, the efficacy of generating tumor in mouse liver is nearly 100%. Liver metastasis burdens are similar among different mice. In addition, the tumor generated by hemispleen models can be easily accessed throughout therapeutic treatments using ultrasound and/or luciferase-expressing KPC cells (Figure 3B,C). In addition, survival is often used as an endpoint for the preclinical studies of experimental therapeutics. If left untreated, mice with hemispleen tumor will die within a short period of time, typically 30–60 days. In the background of immunocompetent mice, these models allow the discovery and preclinical development of novel immunotherapies or combination immunotherapeutics (107).
Conclusions
Different preclinical mouse models provide valuable information on different aspects of PDAC tumor development and therapeutic targets (Table 2). In summary, patient-derived xenograft models allowed close representation of the human disease counterpart by using human specimens while lacking the ability to represent early PDAC development stages, tumor-immune system interaction, as well as the potential for large-scale or high-through drug screening. Humanized mouse models, as alternatives of patient-derived xenograft models, allow the studies of immunotherapeutic targets. Genetically engineered mouse models recapitulate the tumor progression from early PanIN to metastasis and are ideal for most research purposes, but requires labor-intensive and time-consuming efforts. Syngeneic mouse models are efficient for both non-immunotherapeutic and immunotherapeutic studies targeting both primary and/or secondary tumors. It is therefore important to understand the advantages and disadvantages of each model system (Table 1). Multiple tumor models should be combined in a complementary way for each scientific research and drug development project.
Acknowledgments
We thank Keyu Li for providing ultrasound images of animals with PDAC tumors, Dr. Alexandria Surcel and Dr. Amanda Balaban for helpful conversations on the paper organization.
Funding: L.Z. is supported by NCI R01CA169702-06 and R01CA197296.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Colin Weekes) for the series “Systemic and Targeted Therapies for Pancreas Ductal Adenocarcinoma” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2020.03.03). The series “Systemic and Targeted Therapies for Pancreas Ductal Adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. LZ serves as the Editor-in-Chief of Annals of Pancreatic Cancer. LZ reports grants from BMS, grants from Merck, grants from iTeos, grants from Amgen, grants from NovaRock, grants from Inxmed, grants from Halozyme, other from Aduro, personal fees from Biosion, personal fees from Alphamab, personal fees from NovaRock, personal fees from Akrevia, personal fees from Sound Biologics, personal fees from Foundation Medicine, personal fees from Datarevive, from Mingruizhiyao, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. (2020). Cancer Facts and Figures 2020. Available online:https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer 2002;2:897-909. [Crossref] [PubMed]
- Caldas C, Kern SE. K-ras mutation and pancreatic adenocarcinoma. Int J Pancreatol 1995;18:1-6. [PubMed]
- Shields JM, Pruitt K, McFall A, et al. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol 2000;10:147-54. [Crossref] [PubMed]
- Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol 2006;20:211-26. [Crossref] [PubMed]
- Schutte M, Hruban RH, Geradts J, et al. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res 1997;57:3126-30. [PubMed]
- Rozenblum E, Schutte M, Goggins M, et al. Tumor- suppressive pathways in pancreatic carcinoma. Cancer Res 1997;57:1731-4. [PubMed]
- Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nature Reviews Disease Primers 2016;2:16022. [Crossref] [PubMed]
- Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer 2003;3:807-21. [Crossref] [PubMed]
- Ying H, Elpek KG, Vinjamoori A, et al. PTEN Is a Major Tumor Suppressor in Pancreatic Ductal Adenocarcinoma and Regulates an NF-κB–Cytokine Network. Cancer Discov 2011;1:158-69. [Crossref] [PubMed]
- Stanger BZ, Stiles B, Lauwers GY, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell 2005;8:185-95. [Crossref] [PubMed]
- Dougan SK. The Pancreatic Cancer Microenvironment. Cancer J 2017;23:321-5. [Crossref] [PubMed]
- Apte MV, Park S, Phillips PA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 2004;29:179-87. [Crossref] [PubMed]
- Jiang H, Hegde S, DeNardo DG. Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy. Cancer Immunol Immunother 2017;66:1037-48. [Crossref] [PubMed]
- Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013;110:20212-7. [Crossref] [PubMed]
- De Monte L, Reni M, Tassi E, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011;208:469-78. [Crossref] [PubMed]
- Dobrynin YV. Establishment and characteristics of cell strains from some epithelial tumors of human origin. J Natl Cancer Inst 1963;31:1173-95. [PubMed]
- Brunetti O, Russo A, Scarpa A, et al. MicroRNA in pancreatic adenocarcinoma: predictive/prognostic biomarkers or therapeutic targets? Oncotarget 2015;6:23323-41. [Crossref] [PubMed]
- Li P, Xu Q, Zhang D, et al. Upregulated miR-106a plays an oncogenic role in pancreatic cancer. FEBS let 2014;588:705-12.
- Mees ST, Mardin WA, Wendel C, et al. EP300--a miRNA-regulated metastasis suppressor gene in ductal adenocarcinomas of the pancreas. Int J Cancer 2010;126:114-24. [Crossref] [PubMed]
- Gao W, Gu Y, Li Z, et al. miR-615-5p is epigenetically inactivated and functions as a tumor suppressor in pancreatic ductal adenocarcinoma. Oncogene 2015;34:1629-40. [Crossref] [PubMed]
- Deer EL, González-Hernández J, Coursen JD, et al. Phenotype and Genotype of Pancreatic Cancer Cell Lines. Pancreas 2010;39:425-35. [Crossref] [PubMed]
- Rückert F, Aust D, Böhme I, et al. Five primary human pancreatic adenocarcinoma cell lines established by the outgrowth method. J Surg Res 2012;172:29-39. [Crossref] [PubMed]
- Gillet JP, Varma S, Gottesman MM. The clinical relevance of cancer cell lines. J Natl Cancer Inst 2013;105:452-8. [Crossref] [PubMed]
- Froeling FEM, Marshall JF, Kocher HM. Pancreatic cancer organotypic cultures. J Biotechnol 2010;148:16-23. [Crossref] [PubMed]
- Coleman SJ, Watt J, Arumugam P, et al. Pancreatic cancer organotypics: High throughput, preclinical models for pharmacological agent evaluation. World J Gastroenterol 2014;20:8471-81. [Crossref] [PubMed]
- Sereti E, Karagianellou T, Kotsoni I, et al. Patient Derived Xenografts (PDX) for personalized treatment of pancreatic cancer: emerging allies in the war on a devastating cancer? J Proteomics 2018;188:107-18. [Crossref] [PubMed]
- Walters DM, Stokes JB, Adair SJ, et al. Clinical, Molecular and Genetic Validation of a Murine Orthotopic Xenograft Model of Pancreatic Adenocarcinoma Using Fresh Human Specimens. PLoS One 2013;8:e77065. [Crossref] [PubMed]
- Loukopoulos P, Kanetaka K, Takamura M, et al. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas 2004;29:193-203. [Crossref] [PubMed]
- Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A 1992;89:5645-9. [Crossref] [PubMed]
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature 1983;301:527-30. [Crossref] [PubMed]
- Flanagan SP. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genetical research 1966;8:295-309. [Crossref] [PubMed]
- Garcia PL, Council LN, Christein JD, et al. Development and Histopathological Characterization of Tumorgraft Models of Pancreatic Ductal Adenocarcinoma. PLoS One 2013;8:e78183. [Crossref] [PubMed]
- Rubio-Viqueira B, Jimeno A, Cusatis G, et al. An In vivo Platform for Translational Drug Development in Pancreatic Cancer. Clin. Cancer Res 2006;12:4652-61. [Crossref] [PubMed]
- Krempley BD, Yu KH. Preclinical models of pancreatic ductal adenocarcinoma. Chin Clin Oncol 2017;6:25. [Crossref] [PubMed]
- Morton CL, Houghton PJ. Establishment of human tumor xenografts in immunodeficient mice. Nat Protoc 2007;2:247-50. [Crossref] [PubMed]
- Huynh AS, Abrahams DF, Torres MS, et al. Development of an Orthotopic Human Pancreatic Cancer Xenograft Model Using Ultrasound Guided Injection of Cells. PLoS One 2011;6:e20330. [Crossref] [PubMed]
- Pérez-Torras S, Vidal-Pla A, Miquel R, et al. Characterization of human pancreatic orthotopic tumor xenografts suitable for drug screening. Cell Oncol (Dordr) 2011;34:511-21. [Crossref] [PubMed]
- Garber K. From human to mouse and back: 'tumorgraft' models surge in popularity. J Natl Cancer Inst 2009;101:6-8. [Crossref] [PubMed]
- Garrido-Laguna I, Uson M, Rajeshkumar NV, et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res 2011;17:5793-800. [Crossref] [PubMed]
- Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient-derived xenograft mouse models. Nat Rev Cancer 2015;15:311-6. [Crossref] [PubMed]
- Julien S, Merino-Trigo A, Lacroix L, et al. Characterization of a Large Panel of Patient-Derived Tumor Xenografts Representing the Clinical Heterogeneity of Human Colorectal Cancer. Clin Cancer Res 2012;18:5314-28. [Crossref] [PubMed]
- Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002;100:3175-82. [Crossref] [PubMed]
- Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005;174:6477-89. [Crossref] [PubMed]
- Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood 2005;106:1565-73. [Crossref] [PubMed]
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol 2007;7:118-30. [Crossref] [PubMed]
- Walsh NC, Kenney LL, Jangalwe S, et al. Humanized Mouse Models of Clinical Disease. Annu Rev Pathol 2017;12:187-215. [Crossref] [PubMed]
- De La Rochere P, Guil-Luna S, Decaudin D, et al. Humanized Mice for the Study of Immuno-Oncology. Trends Immunol 2018;39:748-63. [Crossref] [PubMed]
- Calvo E, Soria JC, Ma WW, et al. A Phase I Clinical Trial and Independent Patient-Derived Xenograft Study of Combined Targeted Treatment with Dacomitinib and Figitumumab in Advanced Solid Tumors. Clin Cancer Res 2017;23:1177. [Crossref] [PubMed]
- Hussain SM, Reed LF, Krasnick BA, et al. IL23 and TGF-ß diminish macrophage associated metastasis in pancreatic carcinoma. Sci Rep 2018;8:5808. [Crossref] [PubMed]
- Posey AD Jr, Schwab RD, Boesteanu AC, et al. Engineered CAR T Cells Targeting the Cancer- Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity 2016;44:1444-54. [Crossref] [PubMed]
- Raj D, Yang MH, Rodgers D, et al. Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma. Gut 2019;68:1052. [Crossref] [PubMed]
- Ames E, Canter RJ, Grossenbacher SK, et al. NK Cells Preferentially Target Tumor Cells with a Cancer Stem Cell Phenotype. J Immunol 2015;195:4010-9. [Crossref] [PubMed]
- Shultz LD, Brehm MA, Garcia-Martinez JV, et al. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 2012;12:786-98. [Crossref] [PubMed]
- Maleki Vareki S. High and low mutational burden tumors versus immunologically hot and cold tumors and response to immune checkpoint inhibitors. J Immunother Cancer 2018;6:157. [Crossref] [PubMed]
- Haanen JBAG. Converting Cold into Hot Tumors by Combining Immunotherapies. Cell 2017;170:1055-6. [Crossref] [PubMed]
- Hingorani SR, Petricoin Iii EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437-50. [Crossref] [PubMed]
- Herreros-Villanueva M. Mouse models of pancreatic cancer. World J Gastroenterol 2012;18:1286-94. [Crossref] [PubMed]
- Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev 2003;17:3112-26. [Crossref] [PubMed]
- Waters AM, Der CJ. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med 2018;8:a031435. [Crossref] [PubMed]
- Skoulidis F, Cassidy LD, Pisupati V, et al. Germline Brca2 Heterozygosity Promotes KrasG12D -Driven Carcinogenesis in a Murine Model of Familial Pancreatic Cancer. Cancer Cell 2010;18:499-509. [Crossref] [PubMed]
- Lee JW, Komar CA, Bengsch F, et al. Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+) ;LSL-Trp53(R172H/+) ;Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery. Curr Protoc Pharmacol 2016;73:14.39.1-14.39.20.
- Ijichi H, Chytil A, Gorska AE, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev 2006;20:3147-60. [Crossref] [PubMed]
- Guerra C, Schuhmacher AJ, Cañamero M, et al. Chronic Pancreatitis Is Essential for Induction of Pancreatic Ductal Adenocarcinoma by K-Ras Oncogenes in Adult Mice. Cancer Cell 2007;11:291-302. [Crossref] [PubMed]
- Talbert EE, Cuitiño MC, Ladner KJ, et al. Modeling Human Cancer-induced Cachexia. Cell Rep 2019;28:1612-22.e4. [Crossref] [PubMed]
- Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev 2002;12:540-7. [Crossref] [PubMed]
- Schwitzgebel VM. Programming of the pancreas. Mol Cell Endocrinol 2001;185:99-108. [Crossref] [PubMed]
- Slack JM. Developmental biology of the pancreas. Development 1995;121:1569. [PubMed]
- Shih HP, Wang A, Sander M. Pancreas organogenesis: from lineage determination to morphogenesis. Annu Rev Cell Dev Biol 2013;29:81-105. [Crossref] [PubMed]
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development 1996;122:1409. [PubMed]
- Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996;122:983-95. [PubMed]
- Krapp A, Knöfler M, Ledermann B, et al. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev 1998;12:3752-63. [Crossref] [PubMed]
- Kawaguchi Y, Cooper B, Gannon M, et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 2002;32:128-34. [Crossref] [PubMed]
- Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469-83. [Crossref] [PubMed]
- Rowley M, Ohashi A, Mondal G, et al. Inactivation of Brca2 promotes Trp53-associated but inhibits KrasG12D-dependent pancreatic cancer development in mice. Gastroenterology 2011;140:1303-13.e1-3.
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Habbe N, Shi G, Meguid RA, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A 2008;105:18913. [Crossref] [PubMed]
- Murakami S, Nemazanyy I, White SM, et al. A Yap- Myc-Sox2-p53 Regulatory Network Dictates Metabolic Homeostasis and Differentiation in Kras- Driven Pancreatic Ductal Adenocarcinomas. Developmental Cell 2019; [Crossref] [PubMed]
- Rhim AD, Mirek ET, Aiello NM, et al. EMT and Dissemination Precede Pancreatic Tumor Formation. Cell 2012;148:349-61. [Crossref] [PubMed]
- Melo SA, Luecke LB, Kahlert C, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015;523:177. [Crossref] [PubMed]
- Frampton AE, Prado MM, López-Jiménez E, et al. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget 2018;9:19006-13. [Crossref] [PubMed]
- Pérez-Mancera PA, Rust AG, van der Weyden L, et al. The deubiquitinase USP9X suppresses pancreatic ductal adenocarcinoma. Nature 2012;486:266-70. [Crossref] [PubMed]
- Murphy AG, Foley K, Rucki AA, et al. Stromal Annexin A2 expression is predictive of decreased survival in pancreatic cancer. Oncotarget 2017;8:106405-14. [Crossref] [PubMed]
- D’Costa Z, Jones K, Zada A, et al. Gemcitabine-Induced TIMP1 Attenuates Therapy Response and Promotes Tumor Growth and Liver Metastasis in Pancreatic Cancer. Cancer Res 2017;77:5952-62. [Crossref] [PubMed]
- Wong KM, Horton KJ, Coveler AL, et al. Targeting the Tumor Stroma: the Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20). Curr Oncol Rep 2017;19:47. [Crossref] [PubMed]
- Gu D, Schlotman KE, Xie J. Deciphering the role of hedgehog signaling in pancreatic cancer. J Biomed Res 2016;30:353-60. [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Gu D, Liu H, Su GH, et al. Combining hedgehog signaling inhibition with focal irradiation on reduction of pancreatic cancer metastasis. Mol Cancer Ther 2013;12:1038-48. [Crossref] [PubMed]
- Ko AH, LoConte N, Tempero MA, et al. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas 2016;45:370-5. [Crossref] [PubMed]
- Jiang H, Hegde S, Knolhoff BL, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nature Med 2016;22:851-60. [Crossref] [PubMed]
- Bayne LJ, Beatty GL, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell 2012;21:822-35. [Crossref] [PubMed]
- Beatty GL, Winograd R, Evans RA, et al. Exclusion of T Cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extratumoral Macrophages. Gastroenterology 2015;149:201-10. [Crossref] [PubMed]
- Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res 2015;21:3561-8. [Crossref] [PubMed]
- Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612-6. [Crossref] [PubMed]
- Beatty GL, Torigian DA, Chiorean EG, et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res 2013;19:6286-95. [Crossref] [PubMed]
- Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res 2006;12:5277-87. [Crossref] [PubMed]
- Corbett TH, Roberts BJ, Leopold WR, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res 1984;44:717-26. [PubMed]
- Wang Y, Zhang Y, Yang J, et al. Genomic sequencing of key genes in mouse pancreatic cancer cells. Curr Mol Med 2012;12:331-41. [Crossref] [PubMed]
- Torres MP, Rachagani S, Souchek JJ, et al. Novel Pancreatic Cancer Cell Lines Derived from Genetically Engineered Mouse Models of Spontaneous Pancreatic Adenocarcinoma: Applications in Diagnosis and Therapy. PLoS One 2013;8:e80580. [Crossref] [PubMed]
- Lo A, Wang LS, Scholler J, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res 2015;75:2800-10. [Crossref] [PubMed]
- Spear S, Candido JB, McDermott JR, et al. Discrepancies in the Tumor Microenvironment of Spontaneous and Orthotopic Murine Models of Pancreatic Cancer Uncover a New Immunostimulatory Phenotype for B Cells. Front Immunol 2019;10:542. [Crossref] [PubMed]
- Foley K, Rucki AA, Xiao Q, et al. Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer. Sci Signal 2015;8:ra77. -ra. [Crossref] [PubMed]
- Kim VM, Blair AB, Lauer P, et al. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J Immunother Cancer 2019;7:132. [Crossref] [PubMed]
- Soares KC, Foley K, Olino K, et al. A preclinical murine model of hepatic metastases. J Vis Exp. 2014;51677. [PubMed]
- Yanagihara K, Kubo T, Mihara K, et al. Development and Biological Analysis of a Novel Orthotopic Peritoneal Dissemination Mouse Model Generated Using a Pancreatic Ductal Adenocarcinoma Cell Line. Pancreas 2019;48:315-22. [Crossref] [PubMed]
- Blair AB, Kleponis J, Thomas Ii DL, Muth ST, Murphy AG, Kim V, et al. IDO1 inhibition potentiates vaccine-induced immunity against pancreatic adenocarcinoma. J Clin Invest 2019;129:1742-55. [Crossref] [PubMed]
Cite this article as: He M, Henderson M, Muth S, Murphy A, Zheng L. Preclinical mouse models for immunotherapeutic and non-immunotherapeutic drug development for pancreatic ductal adenocarcinoma. Ann Pancreat Cancer 2020;3:7.


