
Paraneoplastic Cushing syndrome caused by a pancreatic neuroendocrine tumor: a case report
Highlight box
Key findings
• We report the case of a 42-year-old female who presented with severe hypokalemia related to a paraneoplastic Cushing syndrome (PCS). Imaging data and histopathological analysis led to the diagnosis of functional well-differentiated pancreatic neuroendocrine tumor (NET) as the source of ectopic adrenocorticotropic hormone (ACTH). She passed away three months after the diagnosis before surgery because of complications particularly pulmonary embolism and severe infections.
What is known and what is new?
• PCS is an uncommon etiology of endogenous hypercortisolism caused generally by bronchial and thymic ACTH-producing tumors. Pancreatic NETs have been reported in only few cases. Patients with PCS are exposed to serious complications.
• Herein, we report a rare case of a young woman in whom Cushing syndrome was caused by ACTH-producing and well-differentiated pancreatic NET.
What is the implication, and what should change now?
• Early identification and surgical removal of the underlying tumor in addition to appropriate management of comorbidities are essential to enhance the prognosis of patients with PCS.
Introduction
Endogenous Cushing syndrome is a rare endocrine disorder that occurs as a result of uncontrolled overproduction of cortisol (1).
Paraneoplastic Cushing syndrome (PCS) is an uncommon cause of endogenous hypercortisolism that represents approximately 15% of all Cushing syndrome (2,3); generally caused by ectopic adrenocorticotropic hormone (ACTH) secretion (EAS) by functional neuroendocrine tumors (NETs) (4,5). PCS is predominantly seen in the context of bronchial carcinoid, thymic carcinoid or medullary thyroid cancer (6). However, EAS in pancreatic NETs has been reported in only few cases (7). Less than 10% of pancreatic NETs arise in the context of hereditary genetic endocrine tumor syndromes (8). In the absence of a unified algorithm, these tumors pose both diagnostic and therapeutic difficulties.
Herein, we report a rare case of a young woman in whom Cushing syndrome was caused by ACTH-producing pancreatic NET.
Case presentation
We present the case of a 42-year-old female who presented with excessive weight gain and generalized muscle weakness. She was hospitalized in the Endocrinology Department of Charles Nicolle Hospital (Tunis) for severe hypokalemia. She had a history of newly diagnosed diabetes mellitus, hypertension, hyperlipidemia, hypothyroidism and hypoparathyroidism after total thyroidectomy for suspicious thyroid nodule which proved to be a macrovesicular adenoma on definitive histology. Clinical examination revealed facial erythrosis, facio-troncular obesity, muscular amyotrophy and elevated blood pressure at 172/85 mmHg.
Initial laboratory evaluation showed severe hypokalemia with a potassium level of 1.7 mEq/L, metabolic alkalosis, normal renal function (creatinine clearance 114.3 mL/mn), high fasting glucose level at 3.2 g/L, lymphopenia (lymphocyte count 350/mm3), eosinopenia (eosinophil count 0/mm3) and a normocytic normochromic anemia with no obvious stigmata of hemolysis.
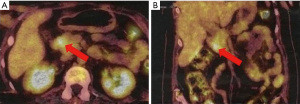
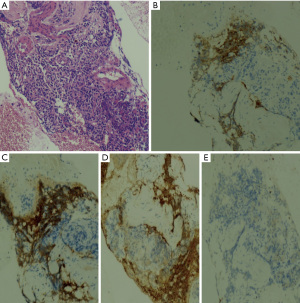
Due to the combination of physical examination findings with newly diagnosed diabetes mellitus, hypertension and severe hypokalemia, the diagnosis of Cushing syndrome was suspected. Hormonal investigations showed elevated urinary free cortisol at 11,048 nmol/day (normal range, 30–197 nmol/day) and serum cortisol at 3,146 nmol/L (normal range, 138–635 nmol/L) with an ACTH level of 126 pg/mL (normal range, 10–60 pg/mL). After 48-hour low-dose dexamethasone test, cortisol remained elevated at 2,226 nmol/L (normal range, >50 nmol/L). Subsequently, pituitary magnetic resonance imaging (MRI) excluded pituitary lesion. Therefore, the possibility of EAS was considered. Cortisol level was not suppressed even with high-dose dexamethasone suppression test thus supporting the diagnosis of EAS. Thoraco-abdominopelvic computed tomography showed just a bilateral adrenal enlargement. A whole-body scintigraphy with 111In-labeled octreotide was negative. However, positron emission tomography with fluorodeoxyglucose (18FDG-PET) revealed a hypermetabolic focus in the pancreatic head (Figure 1). Abdominal MRI was then performed. It detected a hypervascular well-defined mass at the level of pancreatic uncinate process measuring 10 mm × 10 mm highly suspicious for a pancreatic NET with no evidence of distant metastases. Endoscopic ultrasound-guided biopsy of the pancreatic lesion showed a well-differentiated NET with a Ki-67 of less than 1%. On immunohistochemical analysis, tumor cells were positive for chromogranin, synaptophysin and ACTH (Figure 2), confirming the pancreatic tumor as the source of ectopic ACTH. The tumor was graded as grade 1 on World Health Organization (WHO) 2017 classification.
The diagnosis of multiple endocrine neoplasia type 1 was suspected, but it was unlikely. In fact, the other major lesions of this syndrome were missing. Pituitary MRI was normal, especially, it didn’t show a pituitary adenoma and our patient hadn’t primary hyperparathyroidism before thyroidectomy.
After a multidisciplinary meeting, the patient was proposed for duodenopancreatectomy. However, before surgery, she presented acute respiratory distress syndrome related to bilateral massive pulmonary embolism requiring her transfer to intensive care. The pulmonary embolism followed the peripheral venous thrombosis of the right lower limb which was related to the femoral venous catheter to correct the severe hypokalemia despite the patient was on preventive anticoagulation. In addition, our patient developed recurrent respiratory and urinary tract infections treated with antibiotic therapy.
She passed away three months after the diagnosis of PCS despite appropriate multidisciplinary management.
All procedures performed in this report were in accordance with the ethical standards of the national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
EAS is a rare condition found in approximately 10% of patients with ACTH-dependent Cushing syndrome (9,10). It is usually associated with severe and rapid evolving hypercortisolism revealed by variable presentations including cushingoid facial appearance, muscular wasting, hypertensive crisis, hyperglycemia, osteoporosis and severe refractory hypokalemia (9,11-13).
PCS develops as a result of excessive cortisol production induced by extrapituitary ACTH-producing tumors (3,11). PCS may be associated with either highly aggressive malignancies or with slow-growing tumors. According to literature data, the main sources of ectopic ACTH are bronchial carcinoids followed by small-cell lung carcinomas and thymic tumors (11,12,14). ACTH-secreting pancreatic NETs present a rarer source of PCS, reported only in few cases and their incidence is lower than 0.1 per one million people (7,15-22). The majority of reported cases are middle-aged women (23); such as the case of our patient. The mean size of these tumors is 4.43 cm (23). They are more commonly found in the tail of the pancreas and often present with metastatic disease at the time of diagnosis (23). Liver metastases are extremely frequent occurring in 75–88% of patients (7,23). To the best of our knowledge, our patient has the smallest ACTH-secreting pancreatic NET reported so far. Unlike previous reported cases (7,15,18,19,21), the tumor of our patient is located in the head of pancreas with no metastases.
The diagnosis of PCS caused by ACTH-producing NETs is based on vigorous hormonal evaluation (high-dose dexamethasone suppression test, corticotrophin-releasing hormone or desmopressin stimulation test, bilateral petrosal sinus sampling), imaging exploration using pituitary MRI, thoraco-abdominopelvic computed tomography, somatostatin receptor scintigraphy (OctreoScan, Polatom, Poland) and 18FDG-PET, which is far less widely used (17,24); and histological findings (25). The place of 18FDG-PET in localization of ectopic ACTH-secreting tumors is still controversial. Xu et al. (26) reported that the use of integrated 18FDG-PET and computed tomography was useful in the localization of ACTH-secreting tumors in five patients in whom conventional imaging were negative. Surgical removal of detected lesions (FDG uptake) led to normalization of both ACTH and cortisol levels. Moreover, according to a systematic review published by Isidori et al. (27); 18FDG-PET is more sensitive than OctreoScan for detection of abdominal lesions. Our patient had a negative OctreoScan, however, 18FDG-PET was able to locate the lesion.
Several studies showed the effectiveness of endoscopic ultrasonography-guided fine needle aspiration in pancreatic NETs diagnosis and Ki-67 index grading, enabling the assessment of malignancy risk (25,28). Concordance rates between endoscopic ultrasonography-guided fine needle aspiration and resected specimens ranges from 74.0% to 88% (25,28).
Given the complexity and heterogeneity of the disease, the management of ACTH-producing pancreatic NETs is challenging and requires a multidisciplinary approach. The main treatment targets are control of symptomatic hypercortisolism and prolongation of survival. Optimal treatment consists in surgical removal of the pancreatic tumor who can be performed in localized forms and results in full recovery in few cases; however, reported surgical success is modest (30–50%) and late recurrence may occur (17,21). Unfortunately, surgical removal was not performed in our patient because of the burden of comorbidities.
In case of advanced metastatic disease, steroidogenesis inhibitors such as ketoconazole and metyrapone can be administered as the first-line treatment of hypercortisolism (29). These drugs can also be used in preparation for surgery to decrease hypercortisolism morbidity. Bilateral adrenalectomy could be performed for non-resectable pancreatic NETs with severe ectopic ACTH syndrome who do not respond adequately to steroidogenesis inhibitors (15,29). Systemic chemotherapeutic drugs and targeted therapy such as everolimus and sunitinib (tyrosine kinase inhibitor) may be used in patients with advanced pancreatic NETs, although response rates are very low (30).
PCS should be considered as an endocrine emergency as it is associated with considerably high mortality if left untreated. Patients with ACTH-producing pancreatic NETs classically have a poor prognosis (29). Coelho et al. (31) reported that neuroendocrine carcinoma and stage III and IV at diagnosis were independent poor prognostic factors. Prognosis depends mainly on the tumor aggressiveness and the severity of hypercortisolism complications. The 5-year survival rate ranges from 16% to 65% (21,32).
Conclusions
PCS caused by ACTH-secreting pancreatic NETs is a particularly rare condition that heavily compromises patients’ well-being and survival because of multiple comorbidities and unpredictable tumor aggressiveness. Early identification and surgical removal of the tumor, in addition to appropriate management of hypercortisolism complications are essential to reduce morbidity and mortality in these vulnerable patients.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-23-5/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-23-5/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this report were in accordance with the ethical standards of the national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Limumpornpetch P, Morgan AW, Tiganescu A, et al. The Effect of Endogenous Cushing Syndrome on All-cause and Cause-specific Mortality. J Clin Endocrinol Metab 2022;107:2377-88. [Crossref] [PubMed]
- Mineur L, Boustany R, Vazquez L. Paraneoplastic Cushing Syndrome in Gastrointestinal Neuroendocrine Tumour. Case Rep Oncol 2021;14:1407-13. [Crossref] [PubMed]
- Meftah A, Moumen A, Massine El Hammoumi M, et al. Paraneoplastic Cushing's syndrome, a real diagnostic and therapeutic challenge: A case report and literature review. Rev Med Interne 2015;36:843-7. [Crossref] [PubMed]
- Li WY, Liu XD, Li WN, et al. Paraneoplastic Cushing's syndrome associated with bronchopulmonary carcinoid tumor in youth: A case report and review of the literature. Oncol Lett 2016;12:69-72. [Crossref] [PubMed]
- Deldycke A, Haenebalcke C, Taes Y. Paraneoplastic Cushing syndrome, case-series and review of the literature. Acta Clin Belg 2018;73:298-304. [Crossref] [PubMed]
- Torpy DJ, Mullen N, Ilias I, et al. Association of hypertension and hypokalemia with Cushing's syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci 2002;970:134-44. [Crossref] [PubMed]
- Byun J, Kim SH, Jeong HS, et al. ACTH-producing neuroendocrine tumor of the pancreas: a case report and literature review. Ann Hepatobiliary Pancreat Surg 2017;21:61-5. [Crossref] [PubMed]
- Cherchir F, Naceur I, Haouari AA, et al. Unilateral pseudouveitis revealing a pancreatic neuroendocrine carcinoma: A case report. Clin Case Rep 2022;10:e05563. [Crossref] [PubMed]
- Modi A, Olejarski J, Windham A. Severe ectopic Cushing's syndrome. Proc (Bayl Univ Med Cent) 2020;33:601-2. [Crossref] [PubMed]
- Hayes AR, Grossman AB. The Ectopic Adrenocorticotropic Hormone Syndrome: Rarely Easy, Always Challenging. Endocrinol Metab Clin North Am 2018;47:409-25. [Crossref] [PubMed]
- Isidori AM, Lenzi A. Ectopic ACTH syndrome. Arq Bras Endocrinol Metabol 2007;51:1217-25. [Crossref] [PubMed]
- Isidori AM, Kaltsas GA, Pozza C, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J Clin Endocrinol Metab 2006;91:371-7. [Crossref] [PubMed]
- Mohib O, Papleux E, Remmelink M, et al. An ectopic Cushing's syndrome as a cause of severe refractory hypokalemia in the ICU. Acta Clin Belg 2021;76:373-8. [Crossref] [PubMed]
- Davi' MV, Cosaro E, Piacentini S, et al. Prognostic factors in ectopic Cushing's syndrome due to neuroendocrine tumors: a multicenter study. Eur J Endocrinol 2017;176:453-61. [Crossref] [PubMed]
- Lee SL, Ng CY, Sidhu J, et al. A Rare Case of Ectopic Adrenocorticotropic Hormone Secretion from Pancreatic Neuroendocrine Tumour Presenting with Cushing Syndrome. GE Port J Gastroenterol 2023;30:239-42. [Crossref] [PubMed]
- Shayesteh S, Fouladi DF, Fishman EK, et al. Ectopic Cushing syndrome caused by a pancreatic neuroendocrine tumor: A case report. Radiol Case Rep 2020;15:1014-7. [Crossref] [PubMed]
- Wang W, Miao R, Zhang L, et al. An Uncommon Case of Ectopic Adrenocorticotropic Hormone Syndrome from a Pancreatic Neuroendocrine Tumor. Cureus 2019;11:e4076. [Crossref] [PubMed]
- Boustani CH, Von Buttlar X, Zaemes J, et al. S1526 Pancreatic Neuroendocrine Tumor: A Rare Cause of Cushing’s Syndrome. Off J Am Coll Gastroenterol ACG 2020;115:S771-S772.
- do Amor Divino PH, Marchetti KR, Almeida MQ, et al. Functional pancreatic neuroendocrine tumour causing Cushing's syndrome: the effect of chemotherapy on clinical symptoms. Ecancermedicalscience 2017;11:773. [Crossref] [PubMed]
- Miehle K, Tannapfel A, Lamesch P, et al. Pancreatic neuroendocrine tumor with ectopic adrenocorticotropin production upon second recurrence. J Clin Endocrinol Metab 2004;89:3731-6. [Crossref] [PubMed]
- Surace A, Ferrarese A, Benvenga R, et al. ACTH-secreting neuroendocrine pancreatic tumor: a case report. Int J Surg 2014;12:S222-4. [Crossref] [PubMed]
- Vaduganathan M, Nagarur A, Kerr DA, et al. Metastatic pancreatic neuroendocrine tumor with ectopic adrenocorticotropic hormone production. Proc (Bayl Univ Med Cent) 2015;28:46-9. [Crossref] [PubMed]
- Wu Y, Xiong G, Zhang H, et al. Adrenocorticotropic Hormone-Producing Pancreatic Neuroendocrine Neoplasms: A Systematic Review. Endocr Pract 2021;27:152-7. [Crossref] [PubMed]
- Prieto-Tenreiro A, Cabezas-Agrícola JM, Argibay S, et al. The value of 18FDG-PET for localization of ectopic Cushing's syndrome due to occult bronchial carcinoid. Endocrinol Nutr 2011;58:497-9. [Crossref] [PubMed]
- Sugimoto M, Takagi T, Hikichi T, et al. Efficacy of endoscopic ultrasonography-guided fine needle aspiration for pancreatic neuroendocrine tumor grading. World J Gastroenterol 2015;21:8118-24. [Crossref] [PubMed]
- Xu H, Zhang M, Zhai G, et al. The role of integrated (18)F-FDG PET/CT in identification of ectopic ACTH secretion tumors. Endocrine 2009;36:385-91. [Crossref] [PubMed]
- Isidori AM, Sbardella E, Zatelli MC, et al. Conventional and Nuclear Medicine Imaging in Ectopic Cushing's Syndrome: A Systematic Review. J Clin Endocrinol Metab 2015;100:3231-44. [Crossref] [PubMed]
- Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy 2014;46:32-8. [PubMed]
- Cieszyński Ł, Berendt-Obołończyk M, Szulc M, et al. Cushing's syndrome due to ectopic ACTH secretion. Endokrynol Pol 2016;67:458-71. [PubMed]
- Scott AT, Howe JR. Evaluation and Management of Neuroendocrine Tumors of the Pancreas. Surg Clin North Am 2019;99:793-814. [Crossref] [PubMed]
- Coelho S, Costa C, Santos AP, et al. Pancreatic neuroendocrine neoplasms: survival trend analysis of a comprehensive center. Endocr Oncol 2022;2:32-41. [Crossref] [PubMed]
- Handa R, Rahman A. Ectopic ACTH syndrome from metastatic pancreatic neuroendocrine tumor. Consultant 2017;57:472-4.
Cite this article as: Cherchir F, Essayeh S, Mekni S, Ben Hilel W, Gargouri F, Khiari K, Ben Nacef I, Rojbi I. Paraneoplastic Cushing syndrome caused by a pancreatic neuroendocrine tumor: a case report. Ann Pancreat Cancer 2023;6:7.


