
Pancreatic cancer with leptomeningeal carcinomatosis: case report and review of the literature
Highlight box
Key findings
• Patient with leptomeningeal carcinomatosis (LMC) with cervical cord and parenchymal brain involvement secondary to pancreatic cancer.
• Systematic literature review of 25 similar cases.
What is known and what is new?
• LMC in pancreatic cancer is rare but may become more common as patient survival increases.
• Clinical symptoms and imaging characteristics of this patient population as provided by our case report and literature review.
What is the implication, and what should change now?
• As treatments and prognosis improve, the incidence of LMC secondary to pancreatic cancer may rise. Clinicians should be aware of this trend and the developing diagnostic criteria for this rare disease.
Introduction
Leptomeningeal carcinomatosis (LMC) is a complication of advanced malignancy that describes the metastatic spread of a primary cancer to the pia mater, arachnoid mater, and subarachnoid space (1). An estimated 5–8% of solid tumors and 5–15% of hematological cancers are eventually complicated by LMC (2,3). With the greater sensitivity of newer diagnostic modalities and improved efficacy of oncologic treatments over time, LMC has become increasingly prevalent across all primary tumor types (4).
At present, lung, breast, melanoma, acute lymphoblastic leukemia, and non-Hodgkin lymphoma are the most common cancers that give rise to LMC (5). Current management strategies often involve a combination of intra-cerebrospinal fluid (CSF) chemotherapy, systemic therapy, radiotherapy, and supportive measures (6). The prognosis for cancer patients who develop LMC remains poor, however, regardless of tumor type. LMC secondary to pancreatic cancer is exceedingly rare, and there is a paucity of evidence in the literature describing the best evaluation and management strategies for such cases.
In this report, we describe the case of a patient with LMC secondary to pancreatic cancer as well as a systematic review of the relevant literature regarding LMC secondary to pancreatic cancers. We performed a comprehensive literature review using Google Scholar, PubMed, and Ovid Web with the following search terms: ‘leptomeningeal (LM) carcinomatosis’, ‘LM metastasis’, ‘LM disease’, and ‘LM spread’ combined with ‘pancreatic cancer’ or’ ‘tumor’. We included all reports in which an abstract or full manuscript was published in English.
Case presentation
A 72-year-old otherwise healthy Eastern European man presented to the Emergency Department (ED) for several episodes of vomiting and abdominal pain that were initially attributed to gastritis. His symptoms failed to improve with a couple weeks of histamine blockers. One month after his initial presentation, he underwent a comprehensive work-up by gastroenterology, including an esophagogastroduodenoscopy (EGD) which showed excessive fluid in the stomach and moderate extrinsic deformity in the first part of the duodenum and endoscopic ultrasound (EUS) that revealed diffuse enlargement of the pancreatic head. At that time, fine needle aspirate (FNA) taken from the pancreatic head was negative for malignancy.
Due to the inconclusive yet concerning nature of his gastrointestinal (GI) work-up, the patient subsequently underwent exploratory laparotomy, cholecystectomy, Roux-en-Y choledochojejunostomy, gastrojejunostomy, and transduodenal core biopsy of the enlarged pancreatic head approximately 1 month later. In the operating room, the patient was found to have a locally advanced, unresectable pancreatic head mass with involvement of the duodenum and direct extension onto the surface of the gallbladder, as well as encasement of the superior mesenteric vein and portal confluence. Core biopsies revealed poorly differentiated pancreatic ductal adenocarcinoma (T4N0M0) with tumor cells staining positive for CK7, but negative for CK20 and CDX2 markers. Duodenal biopsy showed poorly differentiated carcinoma, and gallbladder showed metastatic poorly differentiated carcinoma involving the gallbladder serosa and surrounding soft tissue.
Soon after the diagnosis of pancreatic adenocarcinoma was confirmed by biopsy, he was started on FOLFIRINOX, but his regimen was complicated by febrile neutropenia, thus precipitating a switch to capecitabine. Approximately 3 months later, he was started on neoadjuvant capecitabine and radiotherapy treatments for 5 weeks. Two months following the conclusion of the capecitabine and radiotherapy treatments, the pancreatic mass appeared to have decreased in size, at which point a Whipple procedure was attempted; however, the mass was again found to be unresectable.
Six months after his last treatment, the patient presented to the ED with severe headache, ataxia, dizziness, and vision changes. Magnetic resonance imaging (MRI) brain showed a right cerebellar contrast-enhancing lesion with extensive surrounding edema (Figure 1). MRI spine showed multi-level, diffuse leptomeningeal changes consistent with advanced metastasis and thus LMC. At this point, given our high suspicion of LMC and the patient’s severe discomfort, we prioritized initiating treatment and controlling the patient’s pain over subjecting the patient to multiple lumbar punctures for the sake of definitive diagnosis.
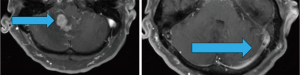
After extensive discussion over goals of care with the patient and his daughter (who acted as an interpreter and medical decision-maker), whole brain radiation therapy (WBRT) was initiated with the aim of maintaining functional independence. He was also started on a steroid regimen of 4 mg dexamethasone every 6 hours with gradual improvement of his neurologic symptoms.
Following treatment, the patient remained ambulatory. However, his clinical course was complicated by bowel perforation requiring endoscopic drain and gastro-jejunal tube placement. He was then referred to hospice care. Oncology continued to follow the patient till about 6 months after the diagnosis of LMC, when his daughter explained by phone that her father’s pain was well-controlled and that he wished to pass peacefully in his home country within Eastern Europe.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Clinical reports of LMC are scarce. This is especially the case with pancreatic cancer. While it is known that LMC occurs more frequently with certain types of tumors, the risk factors that may predispose a patient to developing LMC are not always apparent. Furthermore, with few cases to compare to, clinicians may be unsure of when to initiate diagnostic work-up for the disease. In this case report, we described one case of a male patient who developed LMC in the setting of pancreatic cancer.
This case report represents a valuable contribution to the literature, especially when considering the findings of our literature review. Overall, we identified 23 reports describing only 25 distinct cases of LMC in pancreatic cancer (Table 1) (7-29). Among these reports, analysis of the studies published by Yagi et al. and Hirota et al. were limited since the abstracts were written in English, while the manuscripts were written in Japanese (7,8). One report was completely excluded from review because the data was limited to the abstract and was written completely in Italian (9). The age range of patients included was wide (36–80 years old) and the majority were male (7,10-17,20,24,25,27,29). Interestingly, many patients had no known metastases prior to their diagnosis of LMC (7,13,14,16-22). Like our patient, several patients endorsed headache (7,10,11,15,16,18-26), gait ataxia (14,27,28), and nausea with vomiting (7,15,19,22). Six patients did not receive any treatment whatsoever after diagnosis (16,17,19,29); however, like our patient, 9 different patients underwent WBRT (8,10-12,18,22,25,30) and 7 patients began a steroid regimen (10,13,21,22,27,28,30). Finally, all but 3 (8,25,26) of these patients were deceased by 6 months following LMC diagnosis.
Table 1
| Publication [year] | Age, years/sex | Initial cancer staging | Known metastases prior to diagnosis | Symptoms upon diagnosis of LMC | LMC treatment modalities | Survival following LMC diagnosis (months) |
|---|---|---|---|---|---|---|
| Iwatsuka et al. [2021] | 57/F | T3N1M1 | None | Seizure, headache, nausea, limb numbness, hearing loss | Dexamethasone, nab-PTX + GEM, WBRT | 5 |
| Ceccon et al. [2020] | 51/M | T1NxM1 | L kidney, spleen, lung | Headache, neck stiffness | Gemcitabine + erlotinib, FOLFIRINOX, nab-PTX + GEM | 3 |
| Ikeda et al. [2020] | 59/M | T2NxM1 | Liver | Neck stiffness, lower extremity weakness, dysarthria | None (modified FOLFIRINOX and nab-PTX + GEM prior to dx) | <1 |
| Johnson et al. [2018] | 53/M | T1NxM1 | Liver | Occipital headaches, dysarthria, word-finding difficulty | WBRT, intrathecal topotecan, CAPIRI, bevacizumab | >12 |
| Trinh et al. [2016] | 58/M | None—diagnosed at autopsy | None | Worsening headache, paraparesis, unilateral CNVII palsy | Erroneously treated for TB meningitis given absence of cancer cells in repeat LP | 1 |
| Amico et al. [2016] | 42/F | T3N0M1 | Liver | Headache, neck pain | Steroids, WBRT | 1 |
| Amico et al. [2016] | 57/M | T3N1M1 | Liver, retroperitoneal LN, lungs | Headache, back pain | WBRT | 4 |
| Yoo et al. [2015] | 80/M | T4NxM1 | Liver | Headache, seizure, weight loss | WBRT with palliative intent | Lost to follow-up |
| Naqvi et al. [2015] | 58/F | T1NxM0 | None | Confusion, agitation | Dexamethasone | <1 |
| Hong et al. [2014] | 72/F | T3NxM1 | Liver | Headache, slurred speech, ataxia | Palliative dexamethasone | 8.5 |
| Anne et al. [2013] | 45/F | Unclear; dx was “metastatic adenocarcinoma of unknown primary with pancreatic features” | Various peritoneal implants | Headache, slurred speech, agitation | Palliative care | Lost to follow-up |
| Rao et al. [2013] | 57/M | Poorly differentiated, T3NxM1 | Bone | Seizure, R leg weakness, neck stiffness, photophobia, urinary incontinence | WBRT, palliative RT (spine, R shoulder), FOLFIRINOX† | Transitioned to hospice after 2 cycles of chemotherapy, lost to follow-up |
| Blows et al. [2012] | 72/M | T3N2M1 | Liver, multiple LN | Sudden onset hearing loss, gait ataxia | dexamethasone | <1 |
| Minchom et al. [2010] | 59/M | T2N0M1 | None | Left leg weakness, intermittent seizures | Intrathecal methotrexate, cytarabine, hydrocortisone | <1 |
| Hirota et al. [2008] | 64/M | T3NxMx | None | Vague left-sided pain | WBRT, gemcitabine + S-1 (tegafur, CDHP, Oxo) | 42 |
| Rebischung et al. [2008] | 44/F | T1N1M1 | Superior mesenteric vein | Headache, static instability | methotrexate, thiotepa, MTX-125IUdR | 8 |
| Grira et al. [2007] | 55/M | TxNxM1 | None | Vision loss, vertigo, ataxia | None | 1.5 |
| Yagi et al. [2006] | 64/M | T4NxM1 | None | Fever, vomiting, and headache | Gemcitabine, RT | Lost to follow-up after 20 months of symptom onset |
| Giglio et al. [2005] | 53/F | T3N1M1 | None | Headache, visual complaints | WBRT, doxorubicin, cytoxan | 15 |
| Ferreira Filho et al. [2001] | 49/M | T2N1M1 | Ribs, supraclavicular LN | Headache, vomiting | Intrathecal methotrexate, cytarabine, thiotepa | 1.5 |
| Kurzaj et al. [1980] | 36/M | Acinar cell carcinoma, T3N1M1 | None | Subdural hemorrhage, progressive headache, papilledema, abdominal pain | None—diagnosed at autopsy | 0.5 |
| Little et al. [1974] | 42/M | TxNxM1 | None | Diagnosis established at autopsy | None—diagnosed at autopsy | Unspecified, ranged from 2 weeks to 8 months (average 2 months) |
| Olson et al. [1974] | 46/F | TxNxM1 | Bone | Right hemiparesis, followed by progressive numbness, drowsiness, abnormal behavior, short attention span | Prednisone, WBRT | 1.75 |
| McCormack et al. [1953] | 60/F | T3NxM1 | None | Nausea, vomiting, occipital headaches, hazy vision | None | <2 months of symptom onset |
| McCormack et al. [1953] | 43/F | Undifferentiated, T3N1M1 | None | Back pain, neck pain, L arm numbness, and confusion; then headaches, vomiting, flaccid paralysis, deafness, and blindness | None | 1.25 |
Case reports by Galatioto et al. [1975] and Hirota et al. [2008] were not included in this review because the abstracts were not publicly available for review and both studies were written entirely in either Italian or Japanese, respectively. †, Yagi et al. [2016]—in Japanese, information limited to abstract. LMC, leptomeningeal carcinomatosis; F, female; nab-PTX, nanoparticular albumin-bound paclitaxel; GEM, gemcitabine; WBRT, whole brain radiation therapy; M, male; L, left; FOLFIRINOX, 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin; CAPIRI, capecitabine and irinotecan; CNVII, Cranial Nerve 7; TB, tuberculosis; LP, lumbar puncture; LN, lymph nodes; dx, diagnosis; R, right; RT, radiation therapy.
Based on our literature review alone, the age, sex, and initial pancreatic cancer staging among patients who progress to LMC are highly variable. It is also alarming to note that most patients had no known metastases prior to LMC diagnosis. One previously identified risk factor for eventual LMC has been defined as piecemeal resection of posterior fossa metastases (31,32); however, this finding is limited to patients who had diagnosed brain metastases prior to LMC, and it is not specific to those with primary pancreatic cancer.
Current diagnostic and treatment methods for LMC in general are also scarce. It is generally held that the gold standard of LMC diagnosis is CSF cytology, but the diagnosis is often made in modern practice with the use of MRI (1). Even patients with clear LMC on MRI may have negative cytology, and multiple lumbar punctures are sometimes necessary to solidify diagnosis given the low sensitivity of CSF cytology for malignant cells (33). In a 2020 review article on leptomeningeal metastasis from solid tumors, Thakkar et al. proposed an algorithm for treatment strategies based on risk stratification at the time of diagnosis and clinical presentation. For instance, it is suggested that low risk symptomatic and asymptomatic individuals could benefit from systemic therapy; however, due to the poor prognosis that accompanies leptomeningeal disease, many patients could reasonably pursue symptomatic treatment, along with palliative and comfort care, even at the time of initial diagnosis (34).
This case report and the accompanying literature review are a valuable addition to the literature on this exceedingly rare diagnosis. In fact, the one significant limitation to this study was the sheer lack of existing reports describing LMC in pancreatic cancer. At present, the outlook may seem grim for those diagnosed with LMC. Even so, we suggest that neurologists and neuroradiologists familiarize themselves with the known characteristics of this disease, such that they remain vigilant for new cases.
Conclusions
At present, pancreatic cancer associated LMC is extremely rare, but advancements in the treatment of pancreatic cancer with longer survival rates, and more accurate diagnosis of LMC, will likely lead to a higher incidence of LMC and/or parenchymal involvement for this tumor type. To date, as with LMC associated with other solid tumors, survival is poor.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-23-7/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-23-7/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bhambhvani HP, Rodrigues AJ, Umeh-Garcia MC, et al. Leptomeningeal Carcinomatosis: Molecular Landscape, Current Management, and Emerging Therapies. Neurosurg Clin N Am 2020;31:613-25. [Crossref] [PubMed]
- Nayar G, Ejikeme T, Chongsathidkiet P, et al. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget 2017;8:73312-28. [Crossref] [PubMed]
- Batool A, Kasi A. Leptomeningeal Carcinomatosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; April 5, 2022.
- Rinehardt H, Kassem M, Morgan E, et al. Assessment of Leptomeningeal Carcinomatosis Diagnosis, Management and Outcomes in Patients with Solid Tumors Over a Decade of Experience. Eur J Breast Health 2021;17:371-7. [Crossref] [PubMed]
- Leal T, Chang JE, Mehta M, et al. Leptomeningeal Metastasis: Challenges in Diagnosis and Treatment. Curr Cancer Ther Rev 2011;7:319-27. [Crossref] [PubMed]
- Franzoi MA, Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol 2019;135:85-94. [Crossref] [PubMed]
- Yagi Y, Nishimura Y, Nakatsugawa S, et al. A case of meningeal carcinomatosis from pancreatic cancer during chemotherapy using gemicitabine. Jpn J Gastroenterol Surg 2006;39:1683-88. [Crossref]
- Hirota M, Yagi Y, Yamashita K, et al. A long survival case of unresectable pancreatic cancer by chemoradiotherapy with gemcitabine as key drug. Gan To Kagaku Ryoho 2008;35:2413-6. [PubMed]
- Galatioto S, Savettieri G. Meningeal carcinomatosis secondary to a primary pancreatic tumor. Anatoma-clinical study. Acta Neurol (Napoli) 1975;30:359-67. [PubMed]
- Amico AL, Lukas RV, Kindler HL. Leptomeningeal Carcinomatosis from Pancreatic Cancer Associated with Germline BRCA Mutations: Case Reports and Review of the Literature. Journal of the Pancreas (Online) 2016;17:237-42.
- Yoo IK, Lee HS, Kim CD, et al. Rare case of pancreatic cancer with leptomeningeal carcinomatosis. World J Gastroenterol 2015;21:1020-3. [Crossref] [PubMed]
- Rao R, Sadashiv SK, Goday S, et al. An extremely rare case of pancreatic cancer presenting with leptomeningeal carcinomatosis and synchronous intraparenchymal brain metastasis. Gastrointest Cancer Res 2013;6:90-2. [PubMed]
- Minchom A, Chan S, Melia W, et al. An unusual case of pancreatic cancer with leptomeningeal infiltration. J Gastrointest Cancer 2010;41:107-9. [Crossref] [PubMed]
- Grira MT, Ben Jemaa HM, Lammouchi TM, et al. Meningitis revealing pancreatic carcinoma. Neurosciences (Riyadh) 2007;12:256-8. [PubMed]
- Ferreira Filho AF, Cardoso F, Di Leo A, et al. Carcinomatous meningitis as a clinical manifestation of pancreatic carcinoma. Ann Oncol 2001;12:1757-9. [Crossref] [PubMed]
- Kurzaj E, Kopczynski S, Barowska-Lehman J, et al. Subdural haematoma associated with dural carcinomatosis in a patient with primary carcinoma of pancreas. Neurochirurgia (Stuttg) 1980;23:13-7. [Crossref] [PubMed]
- Little JR, Dale AJ, Okazaki H. Meningeal carcinomatosis. Clinical manifestations. Arch Neurol 1974;30:138-43. [Crossref] [PubMed]
- Giglio P, Weinberg JS, Forman AD, et al. Neoplastic meningitis in patients with adenocarcinoma of the gastrointestinal tract. Cancer 2005;103:2355-62. [Crossref] [PubMed]
- McCormack LJ, Hazard JB, Gardner WJ, et al. Cerebrospinal fluid changes in secondary carcinoma of meninges. Am J Clin Pathol 1953;23:470-8. [Crossref] [PubMed]
- Trinh VT, Medina-Flores R, Chohan MO. Leptomeningeal carcinomatosis as primary manifestation of pancreatic cancer. J Clin Neurosci 2016;30:124-7. [Crossref] [PubMed]
- Naqvi SA, Ahmed I. Carcinomatous Meningitis: A Rare Complication of Pancreatic Adenocarcinoma. J Coll Physicians Surg Pak 2015;25:458-9. [PubMed]
- Iwatsuka K, Kikuta D, Shibuya H, et al. Treatment Outcome of Nab-paclitaxel Plus Gemcitabine for Leptomeningeal Carcinomatosis from Pancreatic Ductal Adenocarcinoma: An Autopsy Case Report. Intern Med 2021;60:3743-8. [PubMed]
- Anne M, Ahmad N, Lee P, et al. An Unusual Presentation of Isolated Leptomeningeal Disease in Carcinoma of Unknown Primary With Pancreatic Features. J Investig Med High Impact Case Rep 2013;1:2324709613494830. [Crossref] [PubMed]
- Ceccon G, Wollring M, Brunn A, et al. Leptomeningeal Carcinomatosis in a Patient with Pancreatic Cancer Responding to Nab-Paclitaxel plus Gemcitabine. Case Rep Oncol 2020;13:35-42. [Crossref] [PubMed]
- Johnson WR, Theeler BJ, Van Echo D, et al. Treatment of Leptomeningeal Carcinomatosis in a Patient with Metastatic Pancreatic Cancer: A Case Report and Review of the Literature. Case Rep Oncol 2018;11:281-8. [Crossref] [PubMed]
- Rebischung C, Hoffmann D, Stefani L, et al. First human treatment of resistant neoplastic meningitis by intrathecal administration of MTX plus (125)IUdR. Int J Radiat Biol 2008;84:1123-9. [Crossref] [PubMed]
- Blows SJ, Morgan R, Dhariwal U, et al. Pancreatic adenocarcinoma presenting with sudden onset bilateral deafness secondary to metastatic leptomeningeal infiltration. Age Ageing 2012;41:818-9. [Crossref] [PubMed]
- Hong CS, Kurt H, Elder JB. Asynchronous leptomeningeal carcinomatosis from pancreatic cancer: a case report and review of the literature. Clin J Gastroenterol 2014;7:434-40. [Crossref] [PubMed]
- Ikeda Y, Yoshida M, Ishikawa K, et al. Pancreatic cancer with leptomeningeal carcinomatosis: case report and literature review. Int Cancer Conf J 2020;9:96-100. [Crossref] [PubMed]
- Olson ME, Chernik NL, Posner JB. Infiltration of the leptomeninges by systemic cancer. A clinical and pathologic study. Arch Neurol 1974;30:122-37. [Crossref] [PubMed]
- Suki D, Abouassi H, Patel AJ, et al. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg 2008;108:248-57. [Crossref] [PubMed]
- Ahn JH, Lee SH, Kim S, et al. Risk for leptomeningeal seeding after resection for brain metastases: implication of tumor location with mode of resection. J Neurosurg 2012;116:984-93. [Crossref] [PubMed]
- Kesari S, Batchelor TT. Leptomeningeal metastases. Neurol Clin 2003;21:25-66. [Crossref] [PubMed]
- Thakkar JP, Kumthekar P, Dixit KS, et al. Leptomeningeal metastasis from solid tumors. J Neurol Sci 2020;411:116706. [Crossref] [PubMed]
Cite this article as: Seu M, Perilla ASP, Melian E, Schneck MJ. Pancreatic cancer with leptomeningeal carcinomatosis: case report and review of the literature. Ann Pancreat Cancer 2023;6:12.


