
Robotic biotissue curriculum for teaching the robotic pancreatoduodenectomy
Introduction
Minimally invasive techniques have revolutionized surgical practice ever since the first laparoscopic appendectomy in 1980 (1) and laparoscopic cholecystectomy in 1985 (2). It has been shown to have decreased short and long-term morbidity and mortality across a wide array of surgical procedures (3-7). Despite the advantages of laparoscopic procedures, their adoption in complex gastrointestinal surgeries such as pancreatoduodenectomy (PD) has been limited to a few centers owing to the advanced technical expertise required (8-11). This left an opening for robotic PD (12-14). Robotic pancreatoduodenectomy (RPD) offers improved three-dimensional imaging, 540° movement of surgical instruments, improved dexterity, and precision in complex tasks like vascular dissection and intracorporeal suturing (15-17).
However, wide-spread adoption of RPD is hindered by a significant learning curve (18-20) and the low volume of surgeries relative to the trainees. Tseng et al. analyzed the learning curve of high volume pancreatic surgeons for open PD and found that perioperative morbidity and mortality improved after 60 cases (21). In a similar study, it was shown than perioperative morbidity and mortality was higher for surgeons who had done less than 50 PDs (22). Compounding the issue further is the lack of standardized programs for safe adoption of this new technique, marking it as a potential safety blind spot for patients (19,23).
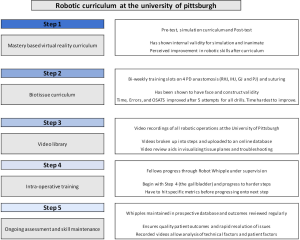
Therefore, it is imperative that a comprehensive and mastery based curriculum be implemented both to shorten the learning curve in RPD and to establish common quality metrics and credentialing systems that help hospitals better gauge the surgical experience of trainees and practicing surgeons. At the University of Pittsburgh, we have developed an innovative comprehensive five step curriculum for RPD that includes a simulation curriculum, a biotissue curriculum, a video library, an operative curriculum and a credentialing system for Society of Surgical Oncology (SSO) and hepato-pancreato-biliary (HPB) fellows (Figure 1).
Mastery-based simulation curriculum
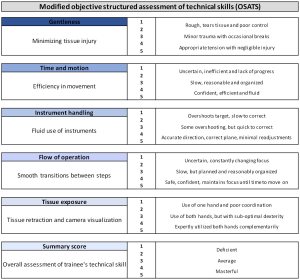
Surgical simulation has advanced significantly over the past two decades with the development of simulators for both laparoscopic and robotic platforms. These have been shown to be valid tools for training and assessment of surgical skill and, more importantly, they have been shown to improve a surgeon’s performance in the operating room (24-27). At the University of Pittsburgh, we have two simulation platforms that are used for trainees. The first is the Intuitive Surgical Backpack Simulator and the second is the Mimic Technologies da Vinci Trainer. On one of these platforms, trainees complete a pre-test which includes four virtual reality exercises and a box-test on the robot with three exercises. Simulated drills were scored by the simulator interface. Inanimate drills on the robot were scored by two trained graders independently according to modified Objective Structured Assessment of Technical Skills (OSATS) (Figure 2) (28,29). Upon completion of these exercises, trainees go through a simulation curriculum on the trainers encompassing 24 virtual reality exercises (Figure 3). This is followed by a post-test at completion which includes the same exercises as a pre-test.
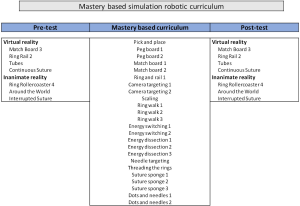
In a previous study published by the group at the University of Pittsburgh, a total of 17 surgical oncology fellows were enrolled in the curriculum and 16 (90%) completed it (30). Of 16 fellows who completed the curriculum, 4 fellows (25%) achieved mastery on all 24 modules with fellows mastering 84% of the modules on average. Individual test scores improved significantly after curriculum completion (P<0.0001) and an average of 2.4 attempts was necessary to master each module. The median time spent completing the curriculum was 4.2 hours across the cohort and, overall, 15 (94%) fellows perceived improvement in robotic skills after completing the curriculum. This showed that a mastery-based simulation curriculum had internal validity with regards to improvement in scores while simultaneously constituting minimal time commitment on the part of the surgical trainee. Having touched on the 1st step of the curriculum, this work will focus on the second step; the biotissue curriculum.
Goals of the biotissue curriculum
Studies have shown equivalence of virtual reality and box simulation for laparoscopic skills (31), our biotissue curriculum addresses the gap in virtual reality training by offering visual feedback on 3-dimensional objects which is especially critical owing to the loss of haptic feedback in the robotic platform (32). In a randomized controlled trial of medical students to compare different methods of learning basic laparoscopic skills using a box trainer, virtual reality simulator and mental training, not all the skills learned in virtual reality were transferable to the box trainer (33). In fact, practice on both the box trainer and the virtual reality simulator has been shown to be important for improvement in laparoscopic skills (34).
Other similar curricula have been reported for additional procedures, though most are short-term and not designed to be proficiency-based with defined metrics and assessment to show improvement over time. Maricic et al. has developed a low-cost inanimate model for minimally invasive repair of esophageal atresia and tracheoesophageal fistula (35). They used different materials to simulate ribs, intercostal spaces, the trachea in addition to different tubular latex balloons to simulate the esophagus. Surgeons of different levels of experience were tasked with testing the model and then answered several questionnaires. In relation to the anatomical characteristics of the model, 94.48% (n=37) of respondents considered that the model has a high degree of similarity; in relation to surgical anatomy 88.2% (n=34) respondents considered that the model has a high degree of similarity; 87.17% (n=34) respondents considered that the model can generate a good amount of skills. Assessment of errors and technical performance showed that there was a significant correlation between surgeon experience and their performance in the model considering operating time (P<0.0001), quality of the anastomosis (P=0.04) and errors (P<0.0001). In another study by Goh et al. evaluated face, content and construct validity of FIRST (Fundamental Inanimate Robotic Skills Tasks), which is a series of four inanimate robotic skills tasks in a large multi-institutional cohort of expert surgeons and trainees (36). Here again, experts appeared to outperform trainees across all skill tasks (P<0.001). Kiely et al. have also developed a low-cost inanimate model of robotic pelvic lymphadenectomy and rated highly for face and content validity (37). Most of these previous studies have validated training models and did not necessarily validate an ongoing curriculum.
It is our group’s assertion that the virtual reality simulator teaches the instrument (clutching, energy switching, using the master controllers and handling the camera), while the biotissue curriculum instills gentle tissue handling and recognition of visual cues and, most importantly, makes the operative steps second nature to the trainee (38). The box trainer is deficient when compared to biotissue owing to the lack of realism in anatomical set up and tissue fidelity (39). Therefore, the steps of our curriculum were designed to progress from one step to another. Simulation is first and this teaches the instrument console, the box trainer is second and this allows trainee to work in an inanimate environment to get a sense of loss of haptics and spatial relations; however, the key component is the deliberate practice biotissue models which mimic the exact step of the corresponding surgical procedure with designated metrics to achieve.
Proving face and construct validity is critical when establishing any new curriculum’s assessment metrics. The biotissue curriculum has been shown to have construct validity because of its ability to distinguish between high and low performance based on measured OSATS, errors and time (35). It also was shown to have face validity when three SSO trained surgeons, who did the drills, rated them as having high levels of likeliness in terms of mechanical set up, tissue fidelity, anatomical angles and needle or suture choice (38).
Methodology of the biotissue curriculum
The bioartificial tissue is created by Lifelike BioTissue Inc. (Ontario, Canada) and the models were designed and assembled by the research team. Fellows are supplied with videos of attending surgeons performing the drills and PowerPoint instructions. Drills are set up on a bi-weekly basis on an Si da Vinci training robot (Intuitive Surgical Inc., Sunnyvale, CA, USA). Fellows are encouraged to sign up, but not mandated.
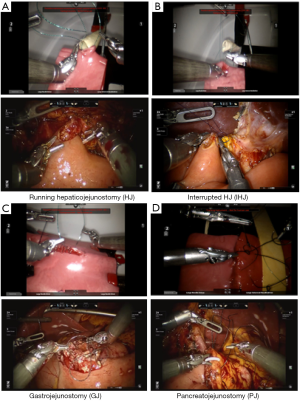
Our biotissue includes two kinds of HJs. The first is a running HJ consisting of one bowel segment cut to 4 cm (acting as jejunum) and a 1 cm wide femoral artery biotissue cut to about 5 cm (acting as a bile duct). The trainee pre-cuts a small hole in the bowel just large enough to anastomose to the “bile duct”. For the running HJ, we supply two running 4-0 vloc stitches (Figure 4A). The interrupted HJ is similar in terms of set up, but uses saphenous vein biotissue instead of the femoral artery biotissue (thinner walled and with smaller diameter). For this drill, we supply the trainees with five 5-0 Maxon stitches cut to 5” (Figure 4B). In the GJ, we use two segments of bowel representing jejunum and stomach cut to around 8 cm. The trainee pre-cuts both bowel segments and then performs a two-layered anastomosis. The trainee is supplied with five 3-0 silk stitches cut to 8” as lambert stitches and two 3-0 vloc as the running and Connell stitches (Figure 4C). And finally, the PJ consists of the same bowel biotissue, but cut to 5-cm long and pancreas biotissue cut to 8-cm wide and 4 cm long. The pancreas biotissue consists of a polymer designed to mimic the actual pancreas, including a pancreatic duct within. The anastomosis performed is a modified Blumgart with five 5-0 Maxon cut to 5” duct-to-mucosa stitches and three 2-0 silk stitches cut to 8” as the outer mattress (Figure 4D).
All anastomotic drills are recorded using AIDA video capture system by KARL STORZ GmbH & Co. KG (Tuttlingen, Germany) and then retrieved by research staff who edit the videos. The research staffs upload the edited video segments to the Vimeo website, developed by Vimeo, Inc. (New York City, New York). The links are sent to crowdsource graders on a weekly basis. These undergraduate hourly employees who are hired at the beginning of each year after passing through training by our research staff. Their training includes having them watch drills completed by experts, novices and moderately proficient surgeons. They are taught to recognize these different skill levels and to grade them according to modified OSATS (Figure 2). The grades are returned a week later and both the video of the drill and these grades are uploaded to a separate Vimeo account and grouped by fellow. The errors and OSATS for the drill are displayed below each video for the fellow to review (Figure 5). Once each fellow has completed a minimum of 5 drills, they begin to receive more detailed report cards on their performance relative to the group.
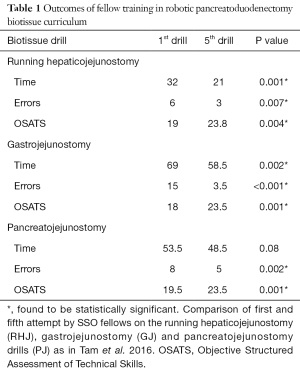
Tam et al. showed that modified OSATS, time and errors improve in fellows who have undergone the biotissue curriculum. On the RHJ and the GJ drills, there was statistically significant decrease in time, errors and OSATS after the fifth attempt (Table 1). On the other hand, while there was a significant improvement in errors and OSATS in the PJ drill after the fifth attempt, there was no significant improvement in time after the fifth attempt. This is likely owing to the difficulty of the PJ anastomosis. The interrupted HJ is a newer drill which has not undergone analysis, yet, but we expect it to mirror the results above.
Full table
The metrics of time, errors, and OSATS for the attending surgeons serve as “mastery” or the expected threshold to achieve for optimal operating room performance. As a group, the trainees were not able to achieve the level of mastery set forth by the attendings for any metric on the running HJ and for time on the other drills. Differentiating individual skill level and performance quartiles to determine factors predictive of better performance is the next step of analysis.
Biotissue curriculum and patient outcomes
The link between technical skill and patient outcomes is well established (28,40). In Birkmeyer et al. the bottom quartile of technical skill, as compared to the top quartile, was associated with higher rates of surgical site infections (4.60% vs. 1.04%; P=0.001), reoperation (3.4% vs. 1.6%; P=0.01), readmission within 30 days (6.3% vs. 2.7%; P<0.001) and higher overall complication rates (14.5% vs. 5.2%; P<0.001). Similarly, the group at the University of Pittsburgh has shown that surgeon operative performance can predict the incidence of post-operative pancreatic fistula (40).
Training using virtual reality simulators and inanimate materials can help improve operative performance. In a recent study by Palter et al., a randomized single-blinded prospective trial allocated 20 surgical trainees to a structured training and assessment curriculum (STAC) group versus conventional residency training. The STAC consisted of case-based learning, proficiency-based virtual reality training, laparoscopic box training, and OR participation. After completion of the intervention, all participants performed 5 sequential laparoscopic cholecystectomies in the OR (41). Residents in the STAC group significantly outperformed residents in the conventional group in the first (P=0.004), second (P=0.036), third (P=0.021), and fourth (P=0.023) surgery. In another study, trainees underwent a validated 16-session advanced laparoscopy simulation training program (42). They were then compared to general surgeons with no simulation training and expert bariatric surgeons in performing a stapled jejunojejunostomy in the OR. They assessed the participants according to the Global rating scale and specific rating scale scores, operative time and the distance traveled by both hands measured with a tracking device. Ten junior trainees, 12 general surgeons and 5 bariatric surgeons were assessed performing a stapled jejunojejunostomy in the OR. All trainees completed the entire anastomosis in the OR without any takeovers by the bariatric surgeons whereas six (50%) bariatric surgeon takeovers took place in the general surgeon group. Trainees had significantly better results in all measured outcomes when compared to general surgeons with considerable higher global rating scale median [19.5 (18.8–23.5) vs. 12 (9–13.8) P<0.001] and lower operative time.
OSATS are reliable and have been repeatedly validated as tools for assessing surgeon technical skill (28,29). Our deliberate biotissue curriculum, in the context of the larger robotics training curriculum at the University of Pittsburgh, improves the technical performance of surgical oncology fellows (38). We are currently in the process of collecting data from the past four years of the curriculum. Our goal is to link trainee participation in the curriculum to increased involvement in operative cases and ultimately better operative performance and improved patient outcomes.
Conclusions
In conclusion, robotic assisted pancreatic surgery improves outcomes and is non-inferior to traditional pancreatic surgery. The lengthy learning curve is the primary barrier against wide-spread implementation of this technique. Utilizing a mastery based robotic curriculum including deliberate practice of the operative steps in the biotissue curriculum can mitigate this learning curve, improve trainee operative involvement and their operative performance. Our group has shown that trainee technical performance improves in terms of time, OSATS and errors. Data directly linking trainee operative performance and practice in the curriculum is currently lacking, but will be detailed in later publications.
Acknowledgements
None.
Footnote
Conflicts of Interest: ME Hogg receives funding from Veteran’s Affairs in way of salary support. AI Al Abbas has no conflicts of interest to declare.


References
- Semm K. Pelviscopic appendectomy. Dtsch Med Wochenschr 1988;113:3-5. [Crossref] [PubMed]
- Reynolds W Jr. The first laparoscopic cholecystectomy. JSLS 2001;5:89-94. [PubMed]
- Aquina CT, Probst CP, Becerra AZ, et al. Missed Opportunity: Laparoscopic Colorectal Resection Is Associated With Lower Incidence of Small Bowel Obstruction Compared to an Open Approach. Ann Surg 2016;264:127-34. [Crossref] [PubMed]
- Gietelink L, Wouters MW, Bemelman WA, et al. Reduced 30-Day Mortality After Laparoscopic Colorectal Cancer Surgery: A Population Based Study From the Dutch Surgical Colorectal Audit (DSCA). Ann Surg 2016;264:135-40. [Crossref] [PubMed]
- McCormack K, Scott NW, Go PM, et al. Laparoscopic techniques versus open techniques for inguinal hernia repair. Cochrane Database Syst Rev 2003.CD001785. [PubMed]
- Shaligram A, Unnirevi J, Simorov A, et al. How does the robot affect outcomes? A retrospective review of open, laparoscopic, and robotic Heller myotomy for achalasia. Surg Endosc 2012;26:1047-50. [Crossref] [PubMed]
- Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. [Crossref] [PubMed]
- Cuschieri A. Laparoscopic surgery of the pancreas. J R Coll Surg Edinb 1994;39:178-84. [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Palanivelu C, Rajan PS, Rangarajan M, et al. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg 2009;16:731-40. [Crossref] [PubMed]
- Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. [Crossref] [PubMed]
- Fonteneau L, Jourdan Da Silva N, Fabre L, et al. Multinational outbreak of travel-related Salmonella Chester infections in Europe, summers 2014 and 2015. Euro Surveill 2017;22:30463. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A 2003;13:33-6. [Crossref] [PubMed]
- Gagner M, Palermo M. Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg 2009;16:726-30. [Crossref] [PubMed]
- Hanly EJ, Talamini MA. Robotic abdominal surgery. Am J Surg 2004;188:19S-26S. [Crossref] [PubMed]
- Zeh HJ 3rd, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg 2011;45:323-40. [Crossref] [PubMed]
- Napoli N, Kauffmann EF, Palmeri M, et al. The Learning Curve in Robotic Pancreaticoduodenectomy. Dig Surg 2016;33:299-307. [Crossref] [PubMed]
- Hogg ME, Besselink MG, Clavien PA, et al. Training in Minimally Invasive Pancreatic Resections: a paradigm shift away from "See one, Do one, Teach one". HPB (Oxford) 2017;19:234-45. [Crossref] [PubMed]
- Boone BA, Zenati M, Hogg ME, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22. [Crossref] [PubMed]
- Tseng JF, Pisters PW, Lee JE, et al. The learning curve in pancreatic surgery. Surgery 2007;141:694-701. [Crossref] [PubMed]
- Schmidt CM, Turrini O, Parikh P, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg 2010;145:634-40. [Crossref] [PubMed]
- Pradarelli JC, Campbell DA Jr, Dimick JB. Hospital credentialing and privileging of surgeons: a potential safety blind spot. JAMA 2015;313:1313-4. [Crossref] [PubMed]
- Ahmed K, Miskovic D, Darzi A, et al. Observational tools for assessment of procedural skills: a systematic review. Am J Surg 2011;202:469-480.e6. [Crossref] [PubMed]
- Andreatta PB, Woodrum DT, Birkmeyer JD, et al. Laparoscopic skills are improved with LapMentor training: results of a randomized, double-blinded study. Ann Surg 2006;243:854-60; discussion 860-3. [Crossref] [PubMed]
- Torkington J, Smith SG, Rees BI, et al. Skill transfer from virtual reality to a real laparoscopic task. Surg Endosc 2001;15:1076-9. [Crossref] [PubMed]
- Wass V, Van der Vleuten C, Shatzer J, et al. Assessment of clinical competence. Lancet 2001;357:945-9. [Crossref] [PubMed]
- Birkmeyer JD, Finks JF, O'Reilly A, et al. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013;369:1434-42. [Crossref] [PubMed]
- Martin JA, Regehr G, Reznick R, et al. Objective structured assessment of technical skill (OSATS) for surgical residents. Br J Surg 1997;84:273-8. [Crossref] [PubMed]
- Hogg ME, Tam V, Zenati M, et al. Mastery-Based Virtual Reality Robotic Simulation Curriculum: The First Step Toward Operative Robotic Proficiency. J Surg Educ 2017;74:477-85. [Crossref] [PubMed]
- Diesen DL, Erhunmwunsee L, Bennett KM, et al. Effectiveness of laparoscopic computer simulator versus usage of box trainer for endoscopic surgery training of novices. J Surg Educ 2011;68:282-9. [Crossref] [PubMed]
- Pinzon D, Byrns S, Zheng B. Prevailing Trends in Haptic Feedback Simulation for Minimally Invasive Surgery. Surg Innov 2016;23:415-21. [Crossref] [PubMed]
- Mulla M, Sharma D, Moghul M, et al. Learning basic laparoscopic skills: a randomized controlled study comparing box trainer, virtual reality simulator, and mental training. J Surg Educ 2012;69:190-5. [Crossref] [PubMed]
- Vitish-Sharma P, Knowles J, Patel B. Acquisition of fundamental laparoscopic skills: is a box really as good as a virtual reality trainer? Int J Surg 2011;9:659-61. [Crossref] [PubMed]
- Maricic MA, Bailez MM, Rodriguez SP. Validation of an inanimate low cost model for training minimal invasive surgery (MIS) of esophageal atresia with tracheoesophageal fistula (AE/TEF) repair. J Pediatr Surg 2016;51:1429-35. [Crossref] [PubMed]
- Goh AC, Aghazadeh MA, Mercado MA, et al. Multi-Institutional Validation of Fundamental Inanimate Robotic Skills Tasks. J Urol 2015;194:1751-6. [Crossref] [PubMed]
- Kiely DJ, Gotlieb WH, Jardon K, et al. Advancing surgical simulation in gynecologic oncology: robotic dissection of a novel pelvic lymphadenectomy model. Simul Healthc 2015;10:38-42. [Crossref] [PubMed]
- Tam V, Zenati M, Novak S, et al. Robotic Pancreatoduodenectomy Biotissue Curriculum has Validity and Improves Technical Performance for Surgical Oncology Fellows. J Surg Educ 2017;74:1057-65. [Crossref] [PubMed]
- Sheckter CC, Kane JT, Minneti M, et al. Incorporation of fresh tissue surgical simulation into plastic surgery education: maximizing extraclinical surgical experience. J Surg Educ 2013;70:466-74. [Crossref] [PubMed]
- Hogg ME, Zenati M, Novak S, et al. Grading of Surgeon Technical Performance Predicts Postoperative Pancreatic Fistula for Pancreaticoduodenectomy Independent of Patient-related Variables. Ann Surg 2016;264:482-91. [Crossref] [PubMed]
- Palter VN, Orzech N, Reznick RK, et al. Validation of a structured training and assessment curriculum for technical skill acquisition in minimally invasive surgery: a randomized controlled trial. Ann Surg 2013;257:224-30. [Crossref] [PubMed]
- Boza C, Leon F, Buckel E, et al. Simulation-trained junior residents perform better than general surgeons on advanced laparoscopic cases. Surg Endosc 2017;31:135-41. [Crossref] [PubMed]
- Al Abbas AI, Hogg ME. First interrupted hepaticojejunostomy (IHJ) of a trainee at the University of Pittsburgh. Asvide 2018;5:060. Available online: http://asvidett.amegroups.com/article/view/22714
- Al Abbas AI, Hogg ME. Seventh interrupted hepaticojejunostomy (IHJ) of a trainee at the University of Pittsburgh. Asvide 2018;5:061. Available online: http://asvidett.amegroups.com/article/view/22715
Cite this article as: Al Abbas AI, Hogg ME. Robotic biotissue curriculum for teaching the robotic pancreatoduodenectomy. Ann Pancreat Cancer 2018;1:9.

