
Radiation induced antitumor autoimmunity: immunotherapies and pancreatic adenocarcinoma
Introduction
With a 5-year survival of 8% overall and 52% of patients with distant disease at presentation, pancreatic adenocarcinoma (ACA) is the fourth leading cause of cancer death for both men and women in the United States (1). Current standard of care chemotherapy regimens for patients with metastatic disease center around a combination of 5-FU, leucovorin, oxaliplatin and irinotecan (FOLFIRINOX) and gemcitabine combined with nab-paclitaxel (2,3) but are only associated with a response rate of up to 30%. Even those patients deemed resectable at initial presentation who undergo surgery and adjuvant therapy have 5-year overall survival rates in the range of 20% with the most favorable subsets of patients undergoing node negative, margin negative (R0) resections at high volume centers still no higher than 39% (4).
Recently, rapid advances in tumor immunology have improved the understanding of key regulators of T cell response and have led to the development of a new immunotherapeutic approach targeting immune checkpoint signaling pathways such as cytotoxic T lymphocyte associated protein 4 (CTLA-4) and programmed death-1 (PD-1). CTLA-4 and PD-1 are negative immune regulators which play an essential role in the immunosuppression of antitumor immunity in the local tumor environment. CTLA-4, expressed on activated T cells, competes with CD28 for binding to B7 on antigen presenting cells to interrupt the costimulatory signal and blunt the T cell response (5). PD-1 is also expressed on the surface of activated T cells. The ligation of PD-1 and PD-L1 (a ligand of PD-1) inhibits T cell proliferation and activation, inducing apoptosis of antigen-specific T cells to prevent collateral tissue damage and autoimmune disease (6). The PD-1/PD-L1 pathway is hijacked by tumor cells to inhibit antitumor immunity, and various cancer cells have been reported to upregulate PD-L1 to escape immune surveillance (7). Several different antibodies blocking these immune checkpoints such as ipilimumab (anti-CTLA-4 antibody), pembrolizumab (anti-PD-1 antibody), nivolumab (anti-PD-1 antibody), atezolizumab (anti-PD-L1 antibody), and durvalumab (anti-PD-L1 antibody) have been extensively studied in a wide spectrum of malignancies. These efforts are rapidly translating into remarkable success of PD-1 blockade agents in melanoma, non-small cell lung cancer, renal cell carcinoma, urothelial cancer, gastric cancer, hepatocellular carcinoma, mismatch repair deficient colorectal cancer and head and neck cancer (8), and immunotherapy has led to a paradigm shift in cancer therapy. However, remarkable success has not been replicated with pancreatic cancer. Several clinical trials of single agent CTLA-4 or PD-L1 antibodies failed to show antitumor activity in patients with locally advanced or metastatic pancreatic cancer (9,10). These data reinforce the concept that pancreatic cancer has a different tumor microenvironment which has direct implications for the integration of immunotherapy agents.
Radiation therapy, one of the main pillars of cancer therapy, plays an essential role in the treatment of a wide variety of cancers. Approximately 60% of patients with solid tumors receive radiation therapy for local disease control (11). Radiation exerts tumoricidal activity through direct DNA damage leading to mitotic catastrophe, apoptosis, necrosis, autophagy and senescence (12). In addition to the direct target effect, radiation can modify the tumor microenvironment, which can lead to systemic antitumor activity at non-irradiated distant sites, a phenomenon known as the abscopal (ab, off; scopus, target) effect which was first described in 1953 (13). It has been believed that the abscopal effect is mediated by radiation induced antitumor immunity (14). In practice, clinical confirmation of the abscopal effect has been rare, with a systematic review evaluating studies reported between 1960 and 2014 reporting only 51 patients as having such responses (14). Barriers to higher rates of the abscopal effect are thought to be secondary to the tumor microenvironment causing local immune suppression characterized by local T-cell inhibition and inadequate priming by dendritic cells (15). Emerging preclinical and clinical data have demonstrated immune-stimulatory effects of radiation which may enhance antitumor immunity of cancer immunotherapy in pancreatic cancer. In this review, the immunomodulatory effects of radiation and the preclinical rationale of the combination of radiation and immunotherapy as a potential treatment strategy in pancreatic cancer will be discussed.
Immunosuppressive tumor microenvironment of pancreatic cancer
The immune system plays a critical role in surveillance against the development and progression of tumors. Pancreatic cancer cells develop several strategies to induce an immunosuppressive tumor microenvironment and evade antitumor immunity in primary and distant metastatic sites, which may contribute to resistance mechanisms of checkpoint immunotherapy in pancreatic cancer.
Primary and metastatic pancreatic cancer cells downregulate major histocompatibility complex (MHC) class I to inhibit tumor antigen cross presentation to cytotoxic T cells (16), which is one of the mechanisms incorporated to escape antitumor immunity. Indoleamine 2,3-dioxygenase (IDO) exhibits an immunosuppressive effect and induces immune tolerance by catabolizing tryptophan which is essential for T cell proliferation (17). High expression of IDO and correlation between IDO expression and poor prognosis were reported in pancreatic cancer (18). In addition, interferon-γ secreted by activated effector T cells for innate and adaptive immune activation upregulated IDO expression, and upregulation of IDO was associated with an increased number of regulatory T cells (Tregs) in metastatic pancreatic cancer (19). Interestingly, it has been suggested that IDO may be a critical resistance mechanism of cancer immunotherapy agents such as ipilimumab, and inhibition of IDO can augment the effectiveness of immunotherapy strategies such as CTLA-4 blockade and PD-1/PD-L1 blockade in a preclinical study (20).
TGF-β is a well-known immunosuppressive cytokine and has direct and indirect immune suppressive effect by inhibiting NK cell mediated cytolysis (21), suppressing CD8 cytotoxic T cell function (22), expanding Tregs and enhancing the function of Tregs (23). Pancreatic cancer cells secrete TGF-β (24) which induce type 2 T helper cell (TH2) immune response associated with tumor growth and reduced survival in patients with pancreatic cancer (25).
To prevent autoimmune disease and minimize collateral damage, activated T cells express Fas, and its ligation with FasL induces apoptosis of activated T cells for immune homeostasis, a process known as activation-induced cell death (26). Pancreatic cancer cells take advantage of activation-induced cell death to escape immune surveillance by expression of FasL on pancreatic cancer cells and induction of apoptosis of cytotoxic T cells (27).
PD-L1, which induces T cell exhaustion and deletion by binding to PD-1 on activated T cells, is expressed on various cancer cells to suppress antitumor immunity in the local tumor microenvironment. A majority of pancreatic cancers also express PD-L1 for immune evasion (28). Immunosuppressive cells such as Tregs, myeloid derived suppressor cells (MDSCs) and tumor associated macrophages (TAMs) play an essential role in inhibition of antitumor immunity (Table 1) (29). Infiltrated immune cells in the tumor and tumor environment of pancreatic cancer are predominantly Tregs, MDSCs and TAMs, and the immunosuppressive cell population is associated with progression of pancreatic cancer (30-32).
Table 1
| Immune cell activity | Immunosuppressive and/or tumorigenic activity |
|---|---|
| Treg suppression of antitumor immunity | • Direct cytotoxicity against effector cells via granzyme and perforin release; |
| • Conversion of ATP, an inflammatory molecule and a danger signal, to inhibitory adenosine by CD39 and CD73 expression; | |
| • Inhibition of maturation of antigen presenting cells and induction of IDO in antigen presenting cells by expression of CTLA-4; | |
| • Consumption of IL-2 via CD25 expression which are essential for T cell proliferation and differentiation; | |
| • Release of immunosuppressive cytokines such as IL-10 and TGF-β. | |
| MDSC Immunosuppressive and tumorigenic activities | • Deprivation of amino acids arginine and cysteine, which are essential for T cell proliferation; |
| • Production of nitric oxide and reactive oxygen species that causes the nitration of T cell receptors and chemokines for preventing T cell migration and inducing apoptosis of T cells and NK cells; | |
| • Production of immunosuppressive cytokines such as IL-10 and TGF-β skewing immune reactions toward Th2 type with activation of Tregs; | |
| • Upregulation of PD-L1 expression which induces T cell exhaustion and deletion; | |
| • Downregulation of TCR ζ-chain expression which are essential for TCR signaling after antigen recognition. | |
| TAM suppression of cytotoxic T Cell response in tumor microenvironment | • Secretion of immunosuppressive IL-10 and TGF-β; |
| • Expression of arginase-1 which suppresses T cell activity by depletion of L-arginine, essential amino acid for T cell function; | |
| • Upregulation of PD-L1; | |
| • Overexpression of IDO. |
MSDC, myeloid derived stem cell; TAM, tumor associated macrophage; Treg, regulatory T cell; IDO, dioxygenase; CTLA-4, cytotoxic T lymphocyte associated protein 4.
Immunomodulatory effects of radiation
Radiation at conventional doses was considered to be immunosuppressive due to the inherent radiosensitivity of immune cells and the attendant normal tissue damage to lymphatic tissue and/or bone marrow secondary to non-conformal older treatment techniques. However, emerging preclinical and clinical data suggest that there are immune-stimulatory effects of radiation. Exposure to radiation can elicit changes in tumor cells and the tumor microenvironment which can enhance the vulnerability of cancer cells to immune attack as shown in Figure 1.
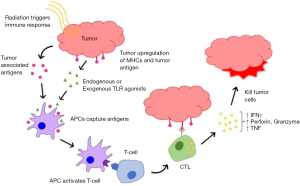
Upregulation of MHC class I molecules and tumor antigens
Radiation enhances tumor antigen presentation to cytotoxic T cells by upregulation of MHC class I molecules. Cell surface expression of MHC class I molecules is increased in a radiation dose dependent manner as a consequence of (I) degradation of existing proteins by radiation, resulting in an increased intracellular peptide pool, (II) enhanced protein synthesis by radiation, resulting also in an increased intracellular peptide pool and (III) increased diversity of the intracellular peptide pool by the radiation induced novel proteins (33). In addition, radiation enhances immunological recognition of cancer cells by tumor specific CD8 T cells through increased expression of tumor specific antigens in tumor cells (34).
Secretion of damage-associated molecular patterns (DAMPs) from tumor cells
Irradiated tumor cells can release DAMPs including calreticulin, adenosine triphosphate (ATP), high mobility group box 1 (HMGB1) and nucleic acids which elicit an antitumor immune response by activation of innate immune systems. Radiation exposure induces translocation of calreticulin from the endoplasmic reticulum to the plasma membrane in cancer cells (35). Calreticulin plays an essential role in the enrichment of endogenous peptides in the endoplasmic reticulum and assembly of MHC class I peptide complex for efficient antigen presentation export (36). Furthermore, the translocation of calreticulin acts as a phagocytic signal (eat-me signal) for antigen presenting cells such as dendritic cells (DC)s (37).
During phagocytosis of irradiated tumor cells in antigen presenting cells, DNA fragments in irradiated tumor cells are released from phagosomes to cytoplasm (cytosolic DNA) (38). Cytosolic DNA induces type I interferons production by activation of stimulator of interferon genes (STING) (39). Type I interferons, bridging the innate immune response to the adaptive response, promotes the cross-priming of cytotoxic T cells and leads to effective tumor growth control.
ATP also stimulates innate immune system by activation of the purinergic receptor P2RX7 which is expressed in immune cells such as macrophages, DCs, monocytes, natural killer cells, B cells and T cells. The activation of P2RX7 by extracellular ATP following tissue damage mediates activation of the innate immune system through the release of pro-inflammatory cytokines such as IL-18 and IL-1β and stimulation of inflammasome and T lymphocyte survival and differentiation (40).
HMGB1 is a soluble protein which is released from dying tumor cells after radiation. HMGB1, which binds to Toll-like receptor 4 (TLR4) on DCs and macrophages, promotes efficient antigen processing and cross presentation of tumor antigen presentations to T cells and induces a potent Th1 cell response by secretion of proinflammatory cytokines such as type I interferons, IL-12, MCP-1, MIP-1α and IP-10 (41).
Effects on regulatory immune cells
Tregs, MDSCs and TAMs are main regulatory immune cells to promote tumor growth and antitumor immune evasion in the tumor microenvironment. The effects of radiation on these regulatory immune cells have not yet been fully elucidated. Previous data demonstrated that Tregs were resistant to radiation induced death, and radiation increased the frequency of Tregs (42,43). However, the effects of radiation on Tregs may be dose dependent. Low dose irradiation (0.94 Gray) induces significant apoptosis of Tregs compared with effector T cells and activates naïve T cells (CD4+CD25−) to express CD25 (44).
Several studies have demonstrated recruitment of MDSCs and TAMs into the tumor microenvironment after radiation treatment (45). Irradiation with a daily dose of 3 Gy for 5 days induces a systemic and local increase of MDSCs and TAMs with elevation of serum CSF1 level (46). Blocking of the CSF1 receptor inhibited migration of MDSCs and TAMs into the tumor microenvironment leading to more effective and durable tumor growth control after local irradiation (46). In addition, a single 15 or 20 Gy fraction of radiation also recruits MDSCs and TAMs to the tumor by overexpression of hypoxia inducible factor-1 (HIF-1) and secretion of stromal derived factor-1 (SDF-1) which recruits MDSCs by binding CXCR4 (47,48). Inhibition of HIF-1 or SDF-1/CXCR4 interaction prevents the influx of MDSCs and TAMs and delayed tumor regrowth (47,48). Interestingly, recent data showed that a single fraction of 12 Gy combined with anti-PD-L1 reduced the local accumulation of MDSCs by cytotoxic actions of TNF against MDSCs from activated CD8 T cells (49).
Radiation can polarize myeloid cells and TAMs to a M2 phenotype which promotes tumor progression and suppresses an antitumor immune response. Irradiation with 15 fractions of 4Gy, 3 fractions of 20 Gy or a single fraction of 25 Gy polarized infiltrated macrophages towards immune suppression which was mediated by transcriptional regulation by NF-κB (50,51). However, it may be a radiation dose dependent manner. A recent study showed that low doses of radiation (≤ 2 Gy) polarized TAMs towards a M1 phenotype with induction of inducible nitric oxide synthase (iNOS), which led to normalization of aberrant vasculature, efficient recruitment of tumor specific T cells and T cell mediated tumor rejection (52).
Clinical role of radiation therapy in pancreatic cancer
Over the last 30 years, radiation treatment techniques have significantly improved with the integration of advanced computer planning and delivery. Treatment has thus become highly conformal, such that the dose can be precisely mapped to the tumor region with a sharp fall off to the normal tissue beyond. Such precision has now evolved to the focal delivery of high dose per fraction treatment in a course of 1 week or less termed stereotactic body radiation therapy (SBRT) (53). As pancreatic cancer moves with breathing (54), escalation of dose is complicated by the proximity of the adjacent normal stomach and duodenum which can change position with organ filling and peristalsis (55). Indeed, when extracranial SBRT techniques were first reported for pancreatic cancer they were typically delivered in a single fraction of 25 Gy (56,57), but maturation of these trials showed an increase in late toxicity with reports of duodenal perforation and stricture; delivery in 5 fractions instead of one fraction reported significantly less late toxicity and no worse local control with current clinical practice favoring multi-fraction treatment (58). With continued advances, the ability to change the treatment plan during a course of treatment (adaptive RT, ART) is now possible, with results showing less duodenum in the high dose range with ART and controlling for the breathing motion with respiratory gating (19%) vs no image guidance nor gating (72%) with a concordant reduction in grade 2 or greater duodenal toxicity from 23% to 7% (59).
The optimal role of radiation therapy (RT) in the pancreatic cancer treatment paradigm is currently not well defined (60). In the adjuvant setting, although early trials showed a survival benefit to regimens containing RT (61,62), these results were not replicated in subsequent European studies (63-65). With the introduction of gemcitabine, an appropriate standard of care became adjuvant gemcitabine with/without capecitabine (66,67) or with appropriately quality assured RT to 50.4 Gy in 28 fractions, biologically effective dose (BED) of 59.47 Gy in patients with a postoperative CA 19 9<90 (68,69). In the neoadjuvant setting, there is data to support RT as part of the regimen to facilitate a margin negative resection, ranging from long course chemoradiation (70,71) to short course SBRT (72,73). In the setting of locally advanced pancreatic cancer, data from a recently published randomized trial did not show a survival advantage to the integration of conventionally fractionated chemoradiation to 54 Gy after systemic therapy (74). This phase III trial data of conventional dosing differed from retrospective data of patients with tumors at least 1 cm away from a luminal GI organ which suggested improved survival and local control if the BED was escalated to 70 Gy (75). In the context of SBRT for locally advanced disease, prospective data in a multi-institutional trial of 6.6 Gy × 5 fractions has shown that RT integration is safe, well tolerated, and associated with a 10% rate of surgical resection (76). With MRI onboard imaging, recent data presented by Rudra et al showed that with ART and dose with a maximum BED >90 Gy, there was a near doubling of local control and overall survival for patients with locally advanced disease (77).
Safe dose escalation in pancreatic cancer is thus of considerable clinical interest. Despite these advances, we are still not able to prospectively personalize the dose of RT for each patient although a number of strategies to integrate radiosensitivity have been described (78). One such model is called the Radiation Sensitivity Index (RSI) which is a 10-gene expression model based on a systems biology approach (79,80). Similarly, a 12-gene model has been developed based on 12 chemokine genes that are immune related and inflammation related called the 12-CK (81,82). Recent data now suggests that RSI and 12-CK are associated and, if combined, may serve as a future pretreatment biomarker to identify individual tumors that would have an increased response to immunotherapy and RT treatment (83). Future prospective trials will need to validate the findings that radiosensitive tumors are more frequently present in tumors with a phenotype of immune activation since these signatures could significantly impact patient selection for treatment modality.
Combination of radiation and immunotherapy
Despite the remarkable success of PD-1 blockade immunotherapy in diverse cancers, a single agent immune checkpoint inhibitor therapy failed to improve the outcome of metastatic pancreatic cancer (84). Several resistance mechanisms of pancreatic ACA to immunotherapy have been suggested. As discussed above, pancreatic cancer has an immunosuppressive tumor microenvironment with downregulation of MHC class I, high expression of IDO, low level of TIL, abundant immune suppressive molecules such as TGF-β and predominant immune suppressive cell population such as Tregs, MDSCs and TAMs. In addition, pancreatic cancer has a low tumor mutation load and low immunogenicity compared with other cancers (85). Tumor mutation burden is associated with neoantigen burden and response to immune checkpoint inhibitor therapy (86,87) since neoantigens can be recognized as non-self by immune cells and elicit cytotoxic T cell immune response. Interestingly, a subset of pancreatic cancers with mismatch repair protein (MMR) deficiency, which is approximately 2% of all pancreatic cancers, (88) harbors greater than 100-fold frameshift and missense mutations compared with MMR proficient tumor (89), and pembrolizumab showed significant anticancer activity in the subset of pancreatic cancer with MMR deficiency (88). Finally, the tumor microenvironment of pancreatic cancer consists of complex and heterogeneous stroma with extracellular matrix protein, cancer associated fibroblasts and endothelial cells, and this dense, fibrotic stroma works as a barrier to effector T cell infiltration (90). To overcome the resistance and improve clinical outcomes, immune checkpoint inhibitors are combined with targeting other immunosuppressive molecules such as LAG-3, TIL-3 and IDO or with chemotherapy, cancer vaccine, T cell therapy or radiation therapy. Here we focus on the combination of immune checkpoint inhibitors and radiation therapy.
Preclinical rationale in solid cancer
The question, then, is how to consider the best way to explore combined RT strategies incorporating immunotherapy to enhance T cell activation and modulate the tumor microenvironment to decrease immunosuppression. Conventionally fractionated radiation relies on the induction of DNA damage to directly kill tumor cells with DNA double-strand breaks leading to mitosis associated cell death or to TP53-mediated apoptosis (91). Doses delivered with stereotactic technique in the 8–10 Gy or higher range have shown higher biological effectiveness, the mechanism of which is still not entirely known, but thought to be related to increased damage to the acid sphingomyelinase apoptotic system of microvascular endothelial cells (92,93). Further, such ablative doses are thought to induce more significant effects on the tumor vasculature, stroma and antitumor immune responses within the local microenvironment, thus causing more cell death (94,95). The question of optimization of dose to achieve this effect is under active investigation since data has shown that a single high dose RT fraction can enhance presentation and T-cell recognition of tumor associated antigens (96). Preclinical data from a murine colorectal tumor model showed that a single dose of 30 Gy, and not fractions of 3 Gy delivered in 10 fractions, was associated with significantly more T cell infiltration in the tumor bed and improved systemic antitumor responses compared with single doses of 15 and 20 Gy (95).
With the enthusiasm for the potential synergy of SBRT and immunotherapy comes a need to further understand the possible mechanisms of interaction. In addition to the immunomodulatory effects of RT discussed above, recent preclinical models have demonstrated the upregulation of PD-L1 by tumors as a response to both fractionated and single high dose RT regimens (49,97), suggesting PD-L1 upregulation may be one of the resistant mechanisms to RT. In the studies, the combination of PD-1/PD-L1 blockade with RT induced higher treatment response and generation of tumor antigen-specific memory immune responses (49,97). Another preclinical study suggests upregulation of PD-L1 expression may be one of the resistance mechanisms of anti-CTLA-4 with RT and dual checkpoint blockade with anti-CTLA-4 and anti-PD-L1 plus RT can reverse the resistance and increase antitumor activity (98). The combination of RT with anti PD-1 therapy has also shown improved local tumor control by upregulation of tumor associated antigen-MHC complexes, enhancement of antigen cross presentation and increased T cell infiltration into tumors in murine melanoma and breast cancer models (96).
Preclinical rationale in pancreatic cancer
Applying this data to pancreatic cancer poses specific challenges due to the highly immunosuppressive tumor microenvironment of pancreatic cancer as discussed above. Furthermore, pancreatic tumors have a lower cumulative mutational load (85) which is associated with poor responses to immunotherapy (86,87). This resulting milieu creates a non-immunogenic tumor microenvironment.
Recent preclinical data suggest the combination of RT, vaccination and checkpoint inhibition may be a new strategy for shifting non-T-cell inflamed pancreatic cancers to T-cell inflamed cancers which respond to immunotherapy (99). In the study, sequential combination of RT, vaccination and PD-L1 blockade enhanced the effector function of tumor infiltrating T cells, leading to significantly improved tumor regression in engineered murine pancreatic cancer expressing SIY antigen to mimic non-inflamed cancer. The findings are provocative, suggesting a new model for converting non-T-cell inflamed cancers to T-cell inflamed cancers with a combination of RT, vaccination and checkpoint blockade. Further preclinical data supports RT’s potential to convert a “cold” pancreatic tumor microenvironment to a “hot” state with data in a mouse pancreatic cancer model showing any combination of an immune checkpoint inhibitor with RT significantly improved overall survival when compared to activity without RT; the best outcome was radiation plus dual checkpoint blockade (anti-CTLA-4 and anti-PD-L1 antibodies) (98).
Ongoing clinical trials of combination of radiation and immunotherapy in resectable, locally advanced or metastatic pancreatic cancer
To date, multiple clinical studies are underway that combine RT with anti-PD-1/PD-L1 (Table 2), RT with anti-PD-1/anti-PD-L1 plus cancer vaccine (Table 2) or with dual checkpoint blockade (anti-CTLA-4 and anti-PD-1/PD-L1) (Table 3) in borderline resectable, locally advanced or metastatic pancreatic cancer. The most common form of radiation therapy in these studies involves high dose SBRT delivery alone, most commonly delivered in multiple fractions. A majority of the studies are the combination of SBRT and immune checkpoint blockade using PD-1/PD-L1 inhibitors to evaluate safety and clinical outcome of the combination in the locally advanced or metastatic disease setting (NCT02648282, NCT02303990, NCT02866383, NCT02311361, and NCT02868632). This combination is also investigated in the neoadjuvant setting with borderline resectable disease (NCT03161379, NCT02305186 and NCT03245541). Although most of the clinical trials are currently in early stages, the results will help characterize the proper fractionation of radiotherapy, dosing and sequencing of treatment for future clinical application.
Table 2
| Malignancy | Immunotherapeutic agent | Radiation fraction | Treatment intent | Phase | Primary endpoint | Clinical trial identifier |
|---|---|---|---|---|---|---|
| Locally advanced pancreatic adenocarcinoma | Pembrolizumab and GVAX | SBRT (6.6 Gy × 5) | Definitive | II | DMFS | NCT02648282 |
| Metastatic melanoma, NSCLC, breast and pancreatic carcinoma | Pembrolizumab | SBRT | Metastatic | I | Number of AEs | NCT02303990 |
| Borderline resectable pancreatic adenocarcinoma | Nivolumab and GVAX pancreas vaccine | SBRT | Neoadjuvant | II | pCR at surgical resection | NCT03161379 |
| Resectable/borderline resectable pancreatic cancer | Pembrolizumab | RT (50.4 × 28) | Neoadjuvant | I/II | Number of TIL, incidence of DLTs | NCT02305186 |
| Borderline resectable and locally advanced pancreatic adenocarcinoma | Durvalumab | SABR (6.6 Gy × 5) | Neoadjuvant/definitive | I/II | Number of DLTs, PFS, Proportion of participants who have resectable disease | NCT03245541 |
*, anti-PD-1/PD-L1 therapeutics included in the table are limited to (A) nivolumab, (B) pembrolizumab or (C) durvalumab. DMFS, distant metastasis free survival; AE, adverse event; pCR, pathologic complete response rate; TIL, tumor infiltrating lymphocytes; DLT, dose-limiting toxicities; PFS, progression free survival; NSCLC, non-small cell lung cancer; SABR, stereotactic ablative radiotherapy; SBRT, stereotactic body radiation therapy; GVAX, granulocyte-macrophage colony-stimulating factor (GM-CSF) gene transfected tumor cell vaccine.
Table 3
| Malignancy | Immunotherapeutic agent | Radiation fraction | Treatment intent | Phase | Primary endpoint | Clinical trial identifier |
|---|---|---|---|---|---|---|
| Metastatic pancreatic carcinoma | Nivolumab or nivolumab + ipilimumab | SBRT (15 Gy × 1) | Metastatic | II | CBR | NCT02866383 |
| MSS and MSI high colorectal cancer, pancreatic cancer | Nivolumab + ipilimumab | Not specified | Metastatic | II | DCR | NCT03104439 |
| Unresectable pancreatic carcinoma | Durvalumab, tremelimumab or durvalumab + tremelimumab | SBRT (8 Gy × 1; 5 Gy × 5) | Definitive | I | AE frequency | NCT02311361 |
| Unresectable and non-metastatic pancreatic cancer | Durvalumab, tremelimumab or durvalumab + tremelimumab | SBRT (6 Gy × 5) | Definitive | I | OS | NCT02868632 |
| Metastatic melanoma, NSCLC, breast cancer, pancreatic adenocarcinoma | Durvalumab + tremelimumab | SBRT (8 Gy × 3 or 17 Gy × 1) | Metastatic | I | Number of AEs | NCT02639026 |
*, anti-PD-1/PD-L1 therapeutics included in the table are limited to (A) nivolumab, (B) pembrolizumab or (C) durvalumab; anti-CTLA-4 therapeutics included in the table are limited to (A) ipilimumab and (B) tremelimumab. CBR, clinical benefit rate; DCR, disease control rate; OS, overall survival; NSCLC, non-small cell lung cancer; SBRT, stereotactic body radiation therapy; AE, adverse event; CTLA-4, cytotoxic T lymphocyte associated protein 4.
Conclusions
Despite the remarkable success of immune checkpoint blockade immunotherapy in diverse cancers, the immunotherapeutic approach has very limited clinical activity to date in pancreatic cancer. Accumulating evidence demonstrates that radiation is a potent immune stimulator which induces antitumor immune response locally and systemically in addition to direct cytotoxic activity. Ablative radiation may have a significant role as part of the therapeutic strategy in combination with immune therapy to convert non-T cell inflamed (“cold”) tumors into highly immunogenic (hot) tumors by upregulation of MHC class I molecules and tumor antigen, secretion of DAMPs, regulation of immunosuppressive cells, and potentially damaging the tumor stroma and microenvironment. However, it is still unclear how to optimally combine radiation and immunotherapy in pancreatic cancer, including optimal sequencing, radiation dose and fractionation to effectively overcome the immunosuppressive pancreatic tumor microenvironment. Completion of ongoing preclinical and clinical studies with the combination of radiation and checkpoint inhibitors is eagerly awaited to answer these questions and may one day improve clinical outcomes in this resilient cancer.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Tuli) for the series “Radiotherapy and Pancreatic Adenocarcinoma” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc.2018.07.04). The series “Radiotherapy and Pancreatic Adenocarcinoma” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. [Crossref] [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [Crossref] [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Topalian SL. Targeting Immune Checkpoints in Cancer Therapy. JAMA 2017;318:1647-8. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother 2010;33:828-33. [Crossref] [PubMed]
- Orth M, Lauber K, Niyazi M, et al. Current concepts in clinical radiation oncology. Radiat Environ Biophys 2014;53:1-29. [Crossref] [PubMed]
- Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol 1999;3:77-83. [Crossref] [PubMed]
- Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol 1953;26:234-41. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]
- Honeychurch J, Cheadle EJ, Dovedi SJ, et al. Immuno-regulatory antibodies for the treatment of cancer. Expert Opin Biol Ther 2015;15:787-801. [Crossref] [PubMed]
- Ryschich E, Notzel T, Hinz U, et al. Control of T-cell-mediated immune response by HLA class I in human pancreatic carcinoma. Clin Cancer Res 2005;11:498-504. [PubMed]
- Mellor AL, Keskin DB, Johnson T, et al. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol 2002;168:3771-6. [Crossref] [PubMed]
- Zhang T, Tan XL, Xu Y, et al. Expression and Prognostic Value of Indoleamine 2,3-dioxygenase in Pancreatic Cancer. Chin Med J (Engl) 2017;130:710-6. [Crossref] [PubMed]
- Witkiewicz A, Williams TK, Cozzitorto J, et al. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J Am Coll Surg 2008;206:849-54; discussion 854-6. [Crossref] [PubMed]
- Holmgaard RB, Zamarin D, Munn DH, et al. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013;210:1389-402. [Crossref] [PubMed]
- Viel S, Marcais A, Guimaraes FS, et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci Signal 2016;9:ra19. [Crossref] [PubMed]
- Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005;8:369-80. [Crossref] [PubMed]
- Marie JC, Letterio JJ, Gavin M, et al. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med 2005;201:1061-7. [Crossref] [PubMed]
- Bellone G, Turletti A, Artusio E, et al. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol 1999;155:537-47. [Crossref] [PubMed]
- De Monte L, Reni M, Tassi E, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011;208:469-78. [Crossref] [PubMed]
- Whiteside TL. Tumor-induced death of immune cells: its mechanisms and consequences. Semin Cancer Biol 2002;12:43-50. [Crossref] [PubMed]
- von Bernstorff W, Spanjaard RA, Chan AK, et al. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery 1999;125:73-84. [Crossref] [PubMed]
- Lu C, Paschall AV, Shi H, et al. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. J Natl Cancer Inst 2017;109. [PubMed]
- Caux C, Ramos RN, Prendergast GC, et al. A Milestone Review on How Macrophages Affect Tumor Growth. Cancer Res 2016;76:6439-42. [Crossref] [PubMed]
- Markowitz J, Brooks TR, Duggan MC, et al. Patients with pancreatic adenocarcinoma exhibit elevated levels of myeloid-derived suppressor cells upon progression of disease. Cancer Immunol Immunother 2015;64:149-59. [Crossref] [PubMed]
- Hiraoka N, Onozato K, Kosuge T, et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006;12:5423-34. [Crossref] [PubMed]
- Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007;67:9518-27. [Crossref] [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [Crossref] [PubMed]
- Sharma A, Bode B, Wenger RH, et al. gamma-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS One 2011;6:e28217 [Crossref] [PubMed]
- Gameiro SR, Jammeh ML, Wattenberg MM, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014;5:403-16. [Crossref] [PubMed]
- Fu H, Liu C, Flutter B, et al. Calreticulin maintains the low threshold of peptide required for efficient antigen presentation. Mol Immunol 2009;46:3198-206. [Crossref] [PubMed]
- Zitvogel L, Kepp O, Senovilla L, et al. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res 2010;16:3100-4. [Crossref] [PubMed]
- Deng L, Liang H, Fu S, et al. From DNA Damage to Nucleic Acid Sensing: A Strategy to Enhance Radiation Therapy. Clin Cancer Res 2016;22:20-5. [Crossref] [PubMed]
- Liang Y, Peng H. STING-cytosolic DNA sensing: the backbone for an effective tumor radiation therapy. Ann Transl Med 2016;4:60. [PubMed]
- Di Virgilio F, Dal Ben D, Sarti AC, et al. The P2X7 Receptor in Infection and Inflammation. Immunity 2017;47:15-31. [Crossref] [PubMed]
- Kim S, Kim SY, Pribis JP, et al. Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med 2013;19:88-98. [Crossref] [PubMed]
- Qu Y, Jin S, Zhang A, et al. Gamma-ray resistance of regulatory CD4+CD25+Foxp3+ T cells in mice. Radiat Res 2010;173:148-57. [Crossref] [PubMed]
- Nakatsukasa H, Tsukimoto M, Tokunaga A, et al. Repeated gamma irradiation attenuates collagen-induced arthritis via up-regulation of regulatory T cells but not by damaging lymphocytes directly. Radiat Res 2010;174:313-24. [Crossref] [PubMed]
- Cao M, Cabrera R, Xu Y, et al. Different radiosensitivity of CD4(+)CD25(+) regulatory T cells and effector T cells to low dose gamma irradiation in vitro. Int J Radiat Biol 2011;87:71-80. [Crossref] [PubMed]
- Vatner RE, Formenti SC. Myeloid-derived cells in tumors: effects of radiation. Semin Radiat Oncol 2015;25:18-27. [Crossref] [PubMed]
- Xu J, Escamilla J, Mok S, et al. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res 2013;73:2782-94. [Crossref] [PubMed]
- Kozin SV, Kamoun WS, Huang Y, et al. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res 2010;70:5679-85. [Crossref] [PubMed]
- Kioi M, Vogel H, Schultz G, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 2010;120:694-705. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]
- Crittenden MR, Cottam B, Savage T, et al. Expression of NF-kappaB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS One 2012;7:e39295 [Crossref] [PubMed]
- Tsai CS, Chen FH, Wang CC, et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys 2007;68:499-507. [Crossref] [PubMed]
- Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589-602. [Crossref] [PubMed]
- Kim SK, Wu CC, Horowitz DP. Stereotactic body radiotherapy for the pancreas: a critical review for the medical oncologist. J Gastrointest Oncol 2016;7:479-86. [Crossref] [PubMed]
- Yoganathan SA, Maria Das KJ, Agarwal A, et al. Magnitude, Impact, and Management of Respiration-induced Target Motion in Radiotherapy Treatment: A Comprehensive Review. J Med Phys 2017;42:101-15. [Crossref] [PubMed]
- Bouchard M, McAleer MF, Starkschall G. Impact of gastric filling on radiation dose delivered to gastroesophageal junction tumors. Int J Radiat Oncol Biol Phys 2010;77:292-300. [Crossref] [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [Crossref] [PubMed]
- Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2008;72:678-86. [Crossref] [PubMed]
- Pollom EL, Alagappan M, von Eyben R, et al. Single- versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: outcomes and toxicity. Int J Radiat Oncol Biol Phys 2014;90:918-25. [Crossref] [PubMed]
- Li XA, Liu F, Tai A, et al. Development of an online adaptive solution to account for inter- and intra-fractional variations. Radiother Oncol 2011;100:370-4. [Crossref] [PubMed]
- Landau E, Kalnicki S. The Evolving Role of Radiation in Pancreatic Cancer. Surg Clin North Am 2018;98:113-25. [Crossref] [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [Crossref] [PubMed]
- Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer 1987;59:2006-10. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Berger AC, Winter K, Hoffman JP, et al. Five-year results of US intergroup/RTOG 9704 with postoperative CA 19-9 </=90 U/mL and comparison to the CONKO-001 trial. Int J Radiat Oncol Biol Phys 2012;84:e291-7. [Crossref] [PubMed]
- Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704--a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 2012;82:809-16. [Crossref] [PubMed]
- Mornex F, Girard N, Scoazec JY, et al. Feasibility of preoperative combined radiation therapy and chemotherapy with 5-fluorouracil and cisplatin in potentially resectable pancreatic adenocarcinoma: The French SFRO-FFCD 97-04 Phase II trial. Int J Radiat Oncol Biol Phys 2006;65:1471-8. [Crossref] [PubMed]
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016;151:e161137 [Crossref] [PubMed]
- Mellon EA, Jin WH, Frakes JM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncol 2017;56:391-7. [Crossref] [PubMed]
- Moningi S, Dholakia AS, Raman SP, et al. The Role of Stereotactic Body Radiation Therapy for Pancreatic Cancer: A Single-Institution Experience. Ann Surg Oncol 2015;22:2352-8. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Krishnan S, Chadha AS, Suh Y, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys 2016;94:755-65. [Crossref] [PubMed]
- Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. [Crossref] [PubMed]
- Rudra S, Jiang N, Rosenberg SA, et al. High Dose Adaptive MRI Guided Radiation Therapy Improves Overall Survival of Inoperable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2017;99:E184 [Crossref]
- Hall WA, Bergom C, Thompson RF, et al. Precision Oncology and Genomically Guided Radiation Therapy: A Report From the American Society for Radiation Oncology/American Association of Physicists in Medicine/National Cancer Institute Precision Medicine Conference. Int J Radiat Oncol Biol Phys 2018;101:274-84. [Crossref] [PubMed]
- Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys 2009;75:489-96. [Crossref] [PubMed]
- Torres-Roca JF, Eschrich S, Zhao H, et al. Prediction of radiation sensitivity using a gene expression classifier. Cancer Res 2005;65:7169-76. [Crossref] [PubMed]
- Coppola D, Nebozhyn M, Khalil F, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol 2011;179:37-45. [Crossref] [PubMed]
- Messina JL, Fenstermacher DA, Eschrich S, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep 2012;2:765. [Crossref] [PubMed]
- Strom T, Torres-Roca JF, Parekh A, et al. Regional Radiation Therapy Impacts Outcome for Node-Positive Cutaneous Melanoma. J Natl Compr Canc Netw 2017;15:473-82. [Crossref] [PubMed]
- O'Reilly EM, Oh D, Dhani N, et al., editors. A randomized phase 2 study of durvalumab monotherapy and in combination with tremelimumab in patients with metastatic pancreatic ductal adenocarcinoma (mPDAC): ALPS study. San Francisco, CA: 2018 Gastrointestinal Cancers Symphosium, 2018.
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Eshleman JR, Lang EZ, Bowerfind GK, et al. Increased mutation rate at the hprt locus accompanies microsatellite instability in colon cancer. Oncogene 1995;10:33-7. [PubMed]
- Watt J, Kocher HM. The desmoplastic stroma of pancreatic cancer is a barrier to immune cell infiltration. Oncoimmunology 2013;2:e26788 [Crossref] [PubMed]
- Radford IR. Evidence for a general relationship between the induced level of DNA double-strand breakage and cell-killing after X-irradiation of mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med 1986;49:611-20. [Crossref] [PubMed]
- Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene 2003;22:5897-906. [Crossref] [PubMed]
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155-9. [Crossref] [PubMed]
- Park HJ, Griffin RJ, Hui S, et al. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012;177:311-27. [Crossref] [PubMed]
- Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res 2015;21:3727-39. [Crossref] [PubMed]
- Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res 2015;3:345-55. [Crossref] [PubMed]
- Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458-68. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Zheng W, Skowron KB, Namm JP, et al. Combination of radiotherapy and vaccination overcomes checkpoint blockade resistance. Oncotarget 2016;7:43039-51. [Crossref] [PubMed]
Cite this article as: Kim DW, Song E, Hoffe S. Radiation induced antitumor autoimmunity: immunotherapies and pancreatic adenocarcinoma. Ann Pancreat Cancer 2018;1:23.

