
Impact of prior cholecystectomy on perioperative outcomes after resection for pancreatic cancer: a single-center, retrospective cohort study in a Chinese population
Introduction
Pancreatic cancer is the seventh leading cause of cancer death in China with an overall 5-year survival rate as low as 7%. The poor prognosis is related to several factors, the most important of which may be the late stage when diagnosed (1). The etiology of pancreatic cancer has not been fully explored. Well-established risk factors for pancreatic cancer include cigarette smoking (2), chronic pancreatitis, diabetes (3), high body mass index (BMI) and centralized fat distribution (4). Moreover, physical inactivity, substance use, and even occupational exposure to certain pesticides are reported to be potential risk factors (5). Although some of the risk factors have been discovered as mentioned above, the etiology of pancreatic cancer has not been fully explored.
Cholecystectomy was reported to be related to increased risk of digestive tract cancer and liver cancer (6,7). There are a few studies that explored the relationship between prior cholecystectomy and pancreatic cancer with contradictory results, Schernhammer et al. (8) reported that subjects with gallstone diseases or cholecystectomy were more likely to have pancreatic cancer than those without. Interestingly, Talamini et al. reported different conclusion (9). A meta-analysis indicated that cholecystectomy is an independent risk factor for pancreatic carcinogenesis recently (10). However, there were significant between-study heterogeneity and modest publication bias in this current meta-analysis. Besides, the data is missing in Chinese mainland population. Therefore, more studies are needed for further elucidation.
Moreover, in a lot of cases, patients with pancreatic cancer who present with biliary symptoms may also undergo improper cholecystectomy and thus delay cancer diagnosis (11). However, it’s uncertain whether prior cholecystectomy is associated with the outcome of patients with pancreatic cancer. The purpose of this study was to demonstrate that prior cholecystectomy may lead to adverse short outcome in patients with pancreatic adenocarcinoma.
Methods
Patients
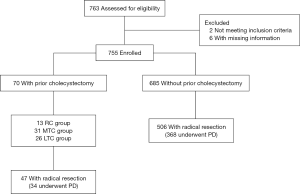
A single-center study was conducted in order to determine the number of patients who had been diagnosed with pancreatic cancer from January 2010 to December 2015 as shown in Figure 1. The study protocol was approved by the review board of The First Affiliated Hospital of Nanjing Medical University. The pathological reports were re-evaluated to rule out patients with non-adenocarcinoma. As a result, 755 patients with pancreatic ductal adenocarcinoma (PDAC) were identified.
Data extraction
Data collected include demographics (age, sex), detailed history (prior surgery, time from cholecystectomy to diagnosis), comorbidities (hypertension, diabetes), intraoperative factors (operation time, estimated blood loss), follow-up vital status, which were acquired from the patients’ clinical notes, operative records, anesthetic charts, and radiologic and pathological reports. Patients with history of cholecystectomy were divided into NPC (no prior cholecystectomy) group and PC (prior cholecystectomy) group. PC group consists of three subgroups: RC (recent cholecystectomy: within 24 months prior to the diagnosis of pancreatic cancer), LTC (long term cholecystectomy: longer than 10 years prior to the diagnosis of pancreatic cancer), MTC (medium term cholecystectomy: longer than 24 months and within 10 years to the diagnosis of pancreatic cancer).
Definitions
All the patients enrolled were initially arranged for surgery. Pancreaticoduodenectomy or distal pancreatectomy with or without vascular reconstruction were performed on resectable or borderline resectable patients. POPF was defined and graded according to criteria proposed by the International Study Group on Pancreatic Fistula (ISGPF) as amylase-rich fluid (more than three-fold greater than the upper limit of serum amylase level) of any measurable volume on or after postoperative day. Delayed gastric emptying (DGE) and postoperative hemorrhage (PPH) were defined and graded using the schema proposed by the International Study Group of Pancreatic Surgery (ISGPS). PPH represented all of the postoperative episodes of hemorrhage.
Statistical analysis
Statistical analyses were performed using SPSS® version 21.0 (IBM, Armonk, New York, USA). Fischer exact, χ2 and t-tests were used to test the significance of differences between patients with cancer undergoing a previous cholecystectomy with those who did not where appropriate. Logistic regression analysis was performed to adjust the P value of risk factors. P<0.05 was considered a significant difference.
Results
Clinical characteristics
Among 755 patients with pathology diagnosis of PDAC, 9.3% (70/755) of the patients underwent prior cholecystectomy (15 days to 30 years), which is more frequent than other prior operations include gynecological operation (42/755), appendectomy (40/755), thyroidectomy (15/755) and mastectomy (7/755) as shown in (Table 1). The rate of prior cholecystectomy in PDAC patients is also significantly higher than that of Chinese population (31/2,579) (Table 2) (12). The baseline clinicopathological characteristics for each group were compared as shown in Tables 3,4. There were 462 men and 293 women were pathologically diagnosed with PDAC. Of the 755 patients, 70 (9.3%) underwent prior cholecystectomy (PC group) and 685 (90.7%) have no history of cholecystectomy (NPC group). There was no significant difference in age, history of alcohol use and smoking, diabetes, AJCC stage and resection rate between PC group and NPC group. There were statistical differences in gender and hypertension between the two groups. Notably, the CA19-9 level was significantly higher in PC group (Table 3). Univariable and multivariable logistic regression analysis for associations between preoperative risk factors and CA19-9 higher than 100 showed that prior cholecystectomy was risk factor for a CA19-9 level higher than 100 (Table 4).
Table 1
| History of surgery | Male (n=462) | Female (n=293) |
|---|---|---|
| Cholecystectomy | 29 | 41 |
| Appendectomy | 25 | 15 |
| Thyroidectomy | 6 | 9 |
| Gynecological operation | – | 42 |
| Mastectomy | – | 7 |
PDAC, pancreatic ductal adenocarcinoma.
Table 2
| History of cholecystectomy | PDAC patients | Chinese population | P |
|---|---|---|---|
| With prior cholecystectomy | 70 (9.3%) | 31 (1.2%) | <0.001 |
| No prior cholecystectomy | 685 (90.7%) | 2,548 (98.8%) (12) |
PDAC, pancreatic ductal adenocarcinoma.
Table 3
| Characteristics | PC group (n=70) | NPC group (n=685) | P |
|---|---|---|---|
| Sex (M/F) | 29/41 | 433/252 | 0.000 |
| Age (y) | 65.6±8.8 | 61.7±10.1 | |
| ≥70 | 20 | 157 | 0.301 |
| <70 | 50 | 528 | |
| Time prior to cholecystectomy | – | ||
| ≥10 years | 26 | – | |
| ≥2 years, <10 years | 31 | – | |
| <2 years | 13 | – | |
| History | |||
| Smoking | 18 | 171 | 0.885 |
| Alcohol | 11 | 114 | 1.000 |
| Comorbidities | |||
| Diabetes | 18 | 121 | 0.107 |
| Hypertension | 20 | 114 | 0.020 |
| AJCC stage | |||
| 1/2 | 41 | 423 | 0.608 |
| 3/4 | 29 | 262 | |
| CA19-9 | |||
| ≥100 | 50 | 397 | 0.030 |
| <100 | |||
| Radical resection | 47 | 506 | 1.000 |
Table 4
| Characteristics | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P | Odds ratio (95% CI) | P | ||
| Sex: male vs. female | 0.86 (0.64–1.16) | 0.320 | |||
| Age: ≥70 vs. <70 | 1.51 (1.06–2.14) | 0.022 | 1.47 (1.04–2.10) | 0.031 | |
| Prior cholecystectomy: yes vs. no | 1.80 (1.05–3.10) | 0.033 | 1.74 (1.01–3.00) | 0.045 | |
| AJCC stage: 3/4 vs. 1/2 | 0.93 (0.69–1.25) | 0.619 | |||
PDAC patients (n=755). PDAC, pancreatic ductal adenocarcinoma.
Operative characteristics and postoperative data
For patients who underwent radical resection, it was suggested that there was no significant difference of operative time and blood loss between LTC/MTC group and NPC group (Table 5). Postoperatively, there was no statistical difference between LTC/MTC group and NPC group in complications as POPF, DGE, hemorrhage and infection. There was also no notably difference in length of hospital stay of these two groups.
Table 5
| Variables | Patients with LTC/MTC | Patients with NPC | P |
|---|---|---|---|
| Operative time (min) | 234.7±108.5 | 235.7±77.7 | 0.714 |
| Blood loss (mL) | 327.6±455.0 | 310.5±276.2 | 0.331 |
| Pancreatic fistula | |||
| Absent | 30 | 433 | 0.268 |
| Present | 8 | 73 | |
| Delayed gastric emptying | |||
| Absent | 33 | 429 | 0.732 |
| Present | 5 | 77 | |
| Hemorrhage | |||
| Absent | 35 | 475 | 0.931 |
| Present | 3 | 31 | |
| Infection | |||
| Absent | 32 | 463 | 0.222 |
| Present | 6 | 43 | |
| Hospital stay (day) | 19.05±6.58 | 20.93±9.94 | 0.368 |
LTC, long term cholecystectomy; MTC, medium term cholecystectomy; NPC, no prior cholecystectomy.
However, RC group had remarkably longer surgery time and more blood loss than NPC group (Table 6) while other parameters as POPF, DGE, hemorrhage and infection and LOS showed no difference. Subgroup analysis for patients who underwent PD (Table 7) showed that the rate of postoperative infection is significantly higher in LTC/MTC patients. However, other characteristics still indicate no remarkable difference.
Table 6
| Variables | Patients with RC | Patients with NPC | P |
|---|---|---|---|
| Operative time (min) | 262.6±83.3 | 235.7±77.7 | 0.164 |
| Blood loss (mL) | 450.00±180.28 | 310.5±276.2 | 0.010 |
| Pancreatic fistula | |||
| Absent | 7 | 433 | 0.857 |
| Present | 2 | 73 | |
| Delayed gastric emptying | |||
| Absent | 7 | 429 | 0.911 |
| Present | 2 | 77 | |
| Hemorrhage | |||
| Absent | 8 | 475 | 1.000 |
| Present | 1 | 31 | |
| Infection | |||
| Absent | 8 | 463 | 0.395 |
| Present | 1 | 43 | |
| Hospital stay (day) | 23.40±6.67 | 20.93±9.94 | 0.110 |
RC, recent cholecystectomy; NPC, no prior cholecystectomy.
Table 7
| Variables | PD patients with LTC/MTC | PD patients with NPC | P |
|---|---|---|---|
| Operative time (min) | 257.9±118.5 | 250.0±79.1 | 0.622 |
| Blood loss (mL) | 398.2±524.5 | 332.9±297.9 | 0.517 |
| Pancreatic fistula | |||
| Absent | 19 | 313 | 0.056 |
| Present | 8 | 55 | |
| Delayed gastric emptying | |||
| Absent | 22 | 300 | 1.000 |
| Present | 5 | 68 | |
| Hemorrhage | |||
| Absent | 25 | 342 | 1.000 |
| Present | 2 | 26 | |
| Infection | |||
| Absent | 21 | 333 | 0.048 |
| Present | 6 | 35 | |
| Hospital stay (day) | 19.48±7.36 | 21.42±10.38 | 0.385 |
PD, pancreaticoduodenectomy; NPC, no prior cholecystectomy; LTC, long term cholecystectomy; MTC, medium term cholecystectomy.
Comparison of pathological characteristics
Five hundred and fifty-three of the 755 patients who underwent radical resection were selected, of whom the pathological data was complete. There is no statistical difference of tumor differentiation, tumor size, nerve invasion and artery invasion between patients with LTC/MTC group versus NPC (Table 8). Patients with prior cholecystectomy had a trend for higher rate of lymph node metastasis than those without. However, no statistical significance was observed. Further subgroup analysis for LTC and MTC group showed no significant difference either (Tables 9,10).
Table 8
| Variables | Patients with LTC/MTC | Patients with NPC | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Histological grade | ||||||
| Well/moderately | 17 | 44.7 | 188 | 37.2 | 0.352 | |
| Poorly | 21 | 55.3 | 318 | 62.8 | ||
| Tumor size | ||||||
| ≤3 cm | 19 | 50 | 242 | 47.8 | 0.796 | |
| >3 cm | 19 | 50 | 264 | 52.2 | ||
| Nerve invasion | ||||||
| Absent | 12 | 31.6 | 127 | 25.1 | 0.377 | |
| Present | 26 | 68.4 | 379 | 74.9 | ||
| Lymph node metastasis | ||||||
| Absent | 14 | 36.8 | 268 | 53.0 | 0.055 | |
| Present | 24 | 63.2 | 238 | 47.0 | ||
| Arterial invasion | ||||||
| Absent | 33 | 86.8 | 435 | 86.0 | 0.881 | |
| Present | 5 | 13.2 | 71 | 14.0 | ||
LTC, long term cholecystectomy; MTC, medium term cholecystectomy; NPC, no prior cholecystectomy.
Table 9
| Variables | Patients with LTC | Patients with NPC | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Histological grade | ||||||
| Well/moderately | 11 | 47.8 | 188 | 37.2 | 0.301 | |
| Poorly | 12 | 52.2 | 318 | 62.8 | ||
| Tumor size | ||||||
| ≤3 cm | 11 | 47.8 | 242 | 47.8 | 0.970 | |
| >3 cm | 12 | 52.2 | 264 | 52.2 | ||
| Nerve invasion | ||||||
| Absent | 9 | 39.1 | 127 | 25.1 | 0.139 | |
| Present | 14 | 60.9 | 379 | 74.9 | ||
| Lymph node metastasis | ||||||
| Absent | 9 | 39.1 | 268 | 53.0 | 0.188 | |
| Present | 14 | 60.9 | 238 | 47.0 | ||
| Arterial invasion | ||||||
| Absent | 21 | 91.3 | 435 | 86.0 | 0.642 | |
| Present | 2 | 8.7 | 71 | 14.0 | ||
LTC, long term cholecystectomy; NPC, no prior cholecystectomy.
Table 10
| Variables | Patients with MTC | Patients with NPC | P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Histological grade | ||||||
| Well/moderately | 8 | 53.3 | 188 | 37.2 | 0.453 | |
| Poorly | 7 | 46.7 | 318 | 62.8 | ||
| Tumor size | ||||||
| ≤3 cm | 8 | 53.3 | 242 | 47.8 | 0.696 | |
| >3 cm | 7 | 46.7 | 264 | 52.2 | ||
| Nerve invasion | ||||||
| Absent | 3 | 20.0 | 127 | 25.1 | 0.870 | |
| Present | 12 | 80.0 | 379 | 74.9 | ||
| Lymph node metastasis | ||||||
| Absent | 5 | 33.3 | 268 | 53.0 | 0.130 | |
| Present | 10 | 66.7 | 238 | 47.0 | ||
| Arterial invasion | ||||||
| Absent | 12 | 80.0 | 435 | 86.0 | 0.817 | |
| Present | 3 | 20.0 | 71 | 14.0 | ||
MTC, medium term cholecystectomy; NPC, no prior cholecystectomy.
Discussion
This study reports a surprisingly high incidence of cholecystectomies prior to diagnosis of pancreatic cancer in Chinese mainland population for the first time. Cholecystectomy is extensively performed in Chinese patients because of high incidence of gallstone and the great safety and feasibility of laparoscopic cholecystectomy. For patients with pancreatic cancer, the rate is even higher. Part of the reason is that symptoms such as upper abdominal pain or even jaundice can concur with frequent diseases such as cholecystolithiasis. Moreover, laparoscopic cholecystectomy is the treatment of choice for the vast majority of patients with symptomatic cholecystolithiasis. However, a major drawback remains the lack of visceral palpation of the abdominal organs. Indeed, several case reports have indicated that this disadvantage of laparoscopic cholecystectomy can lead to missing diagnosis and a fatal delay in the diagnosis of abdominal malignancies (11,13,14). In addition to missing diagnosis, presenting signs and symptoms including jaundice, abdominal pain, nausea, and dark urine could be simply caused by PDAC instead of cholecystolithiasis where misdiagnosis could be involved. A recent study showed that initial misdiagnosis of patients with proximal PDAC is associated with delay in diagnosis and higher risk of locally advanced or advanced disease at time of PDAC diagnosis (15). Also, longer time from symptom onset to diagnosis has been shown to be an independent risk factor for survival in a previous study (16). Therefore, the missing diagnosis and (or) misdiagnosis may happen in PDAC patients with recent cholecystectomy. But whether long term history of cholecystectomy can affect the progression and prognosis in PDAC patients is still uncertain although some studies have given vague answers (17,18).
In this study, we found that patients with prior cholecystectomy may have more chance for the invasion of lymph node, which is strongly associated with adverse outcome. Patients with prior cholecystectomy have higher level of serum CA19-9, which is also an important prognostic factor for the survival of PDAC patients. Therefore, these two points give us confidence that the history of cholecystectomy may be an adverse prognostic factor for PDAC (19,20).
The mechanism behind this fact is still unclear. Animal models suggest that cholecystectomy can promote the proliferation of pancreatic acinar cells by increased the level of cholecystokinin (CCK). On the other hand, it has been suggested that metabolism of bile salts is enhanced after cholecystectomy. Secondary bile acids or metabolites may have carcinogenic effects on the colon, liver, and pancreas (21).
In addition, cholecystectomy may suppress the normal inhibitory effect of CCK on the sphincter of Oddi (22). The presence of gallstones, on the other hand, appears to be associated with chronic pancreatitis (23), which increase the risk of pancreatic cancer. However, the strength of the association remains uncertain because of the retrospective design of most analyses.
The difference of the operative characteristics and postoperative data among the subgroups is not very notable. However, it indicated that patients with recent cholecystectomy seem to have more blood loss in operation, which is not hard to conceive because a recent operation obviously makes the resection even more difficult. The lack of visceral palpation during laparoscopic cholecystectomy presents an imminent problem for laparoscopic surgery in general and might be one reason for the undetected pancreatic cancers in our study. In addition, there is also a weak difference in the postoperative infection rate between LTC group and NPC group who had PD for treatment. However, we cannot rule out the possibility of the bias of this study for this discovery.
A limitation of this study was that we only analyzed patients from one single surgical center. Perhaps, some patients’ status post recent cholecystectomy were not amenable to curative resection secondary to local invasion or metastatic disease and thus were excluded from our analysis. Also, the survival information is absent in this study due to the unfinished follow up, which should have improved the quality for this research.
Conclusions
This report demonstrates that there is an abnormally high proportion of patients with cholecystectomy history in patients with pancreatic cancer in Chinese population, which needs validation in further epidemiological studies. Prior cholecystectomy may result in substantially higher level of serum CA19-9 when diagnosed and higher rate of lymph node invasion which may compromise the prognosis and survival of PDAC patients. Also, PDAC patients with recent cholecystectomy history may have adverse outcome because of the delay of diagnosis and the higher rate of complications caused by higher degree of difficulty for surgery, which calls for more caution for elderly patients with upper abdominal pain or jaundice.
Acknowledgments
Funding: This study was funded by the National Natural Science Foundation of China (No. 81672449), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231801), Jiangsu Key Medical Discipline (General Surgery, ZDXKA2016005), the Natural Science Foundation of Jiangsu Province (BK20161590), the Innovation Capability Development Project of Jiangsu Province (BM2015004), and the Talents Planning of Six Summit Fields of Jiangsu Province (WSW-019).
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Yingbin Liu and Wei Gongi) for the series “The 8th Annual International Surgery Forum” published in Annals of Pancreatic Cancer. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ apc.2019.03.01). The series “The 8th Annual International Surgery Forum” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the review board of The First Affiliated Hospital of Nanjing Medical University (No. 2016-SR-094). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Wang L, Kong L, Wu F, et al. Preventing chronic diseases in China. Lancet 2005;366:1821-4. [Crossref] [PubMed]
- Ferlay J, Burkhard C, Whelan S, Parkin DM. Check and conversion programs for cancer registries (IACR/IACR Tools for Cancer Registries). IACR Technical Report No. 42. Lyon: 2005.
- Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170:791-802. [Crossref] [PubMed]
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- Shao T, Yang YX. Cholecystectomy and the risk of colorectal cancer. Am J Gastroenterol 2005;100:1813-20. [Crossref] [PubMed]
- Liu Y, He Y, Li T, et al. Risk of primary liver cancer associated with gallstones and cholecystectomy: a meta-analysis. PLoS One 2014;9:e109733 [Crossref] [PubMed]
- Schernhammer ES, Michaud DS, Leitzmann MF, et al. Gallstones, cholecystectomy, and the risk for developing pancreatic cancer. Br J Cancer 2002;86:1081-4. [Crossref] [PubMed]
- Talamini G, Falconi M, Bassi C, et al. Previous cholecystectomy, gastrectomy, and diabetes mellitus are not crucial risk factors for pancreatic cancer in patients with chronic pancreatitis. Pancreas 2001;23:364-7. [Crossref] [PubMed]
- Fan Y, Hu J, Feng B, et al. Increased Risk of Pancreatic Cancer Related to Gallstones and Cholecystectomy: A Systematic Review and Meta-Analysis. Pancreas 2016;45:503-9. [Crossref] [PubMed]
- Ghadimi BM, Horstmann O, Jacobsen K, et al. Delay of diagnosis in pancreatic cancer due to suspected symptomatic cholelithiasis. Scand J Gastroenterol 2002;37:1437-9. [Crossref] [PubMed]
- Zhou ZY, Wang Y, Han Y. The Relationship between Cholecystectomy and Colorectal Cancer in China: a Meta-analysis. Clin J Med Offic 2007;35:343-5.
- Slim K, Pezet D, Clark E, et al. Malignant tumors missed at laparoscopic cholecystectomy. American Journal of Surgery 1996;171:364-5. [Crossref] [PubMed]
- Malouf AJ, Murray AW, Macgregor MAB. Major intra-abdominal pathology missed at laparoscopic cholecystectomy. Br J Surg 2000;87:1434. [Crossref] [PubMed]
- Swords DS, Mone MC, Zhang C, et al. Initial Misdiagnosis of Proximal Pancreatic Adenocarcinoma Is Associated with Delay in Diagnosis and Advanced Stage at Presentation. J Gastrointest Surg 2015;19:1813-21. [Crossref] [PubMed]
- Gobbi PG, Bergonzi M, Comelli M, et al. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol 2013;37:186-90. [Crossref] [PubMed]
- Gray SH, Hawn MT, Kilgore ML, et al. Does cholecystectomy prior to the diagnosis of pancreatic cancer affect outcome? Am Surg 2008;74:602-5. [PubMed]
- Schiffman SC, Chu CK, Park J, et al. Is prior cholecystectomy associated with decreased survival in patients with resectable pancreatic adenocarcinoma following pancreaticoduodenectomy? Am J Surg 2011;201:519-24. [Crossref] [PubMed]
- Chen Y, Gao SG, Chen JM, et al. Serum CA242, CA199, CA125, CEA, and TSGF are Biomarkers for the Efficacy and Prognosis of Cryoablation in Pancreatic Cancer Patients. Cell Biochem Biophys 2015;71:1287-91. [Crossref] [PubMed]
- Paiella S, Sandini M, Gianotti L, et al. The prognostic impact of para-aortic lymph node metastasis in pancreatic cancer: A systematic review and meta-analysis. Eur J Surg Oncol 2016;42:616-24. [Crossref] [PubMed]
- Ura H, Makino T, Ito S, et al. Combined Effects of Cholecystectomy and Lithocholic Acid on Pancreatic Carcinogenesis of N-Nitrosobis(2-hydroxypropyl)amine in Syrian Golden Hamsters. Cancer Res 1986;46:4782-6. [PubMed]
- Luman W, Williams AJ, Pryde A, et al. Influence of cholecystectomy on sphincter of Oddi motility. Gut 1997;41:371-4. [Crossref] [PubMed]
- Hardt PD, Bretz L, Krauss A, et al. Pathological pancreatic exocrine function and duct morphology in patients with cholelithiasis. Dig Dis Sci 2001;46:536-9. [Crossref] [PubMed]
Cite this article as: Yin L, Liu X, Liu T, Fu Y, Peng Y, Ding D, Lu Z, Gao W, Wu J, Jiang K, Wei J, Miao Y. Impact of prior cholecystectomy on perioperative outcomes after resection for pancreatic cancer: a single-center, retrospective cohort study in a Chinese population. Ann Pancreat Cancer 2019;2:4.

