
MRI screening in hereditary pancreatic cancer: value of various sequences in the detection of early pancreatic cancer
Introduction
Despite decades of advances in research and treatment, pancreatic ductal adenocarcinoma (PDAC) remains a lethal disease with an overall 5-year survival rate of only 8% (1). The majority of symptomatic patients are incurable, with tumors diagnosed at an advanced stage. Therefore, there is a strong interest in detecting precursor lesions and small asymptomatic cancers that are potentially curable. Given the overall low incidence of PDAC and the lack of accurate, inexpensive, and non-invasive diagnostic tests for early detection, a widespread screening program does not seem feasible or cost-effective.
However, screening may be desirable in selected high risk individuals. PDAC has been shown to have a hereditary predisposition with estimates ranging from 3% to 16% of newly diagnosed cases (2-4), varying between known germline mutations and a positive family history for pancreatic cancer.
In a recent multicentre study, annual MRI surveillance of a large cohort of CDKN2A/p16-Leiden mutation carriers [lifetime risk of pancreatic cancer 15–20% (5)] resulted in a higher resection rate of screen-detected PDAC compared with symptomatic pancreatic cancer (6,7).
Recently, more results of surveillance and recommendations for screening of high risk individuals have been published (8,9). Most authors recommend a surveillance program using MRI, endoscopic ultrasonography (EUS) or combining both modalities. MRI and EUS may be complementary to each other, rather than interchangeable (10). Most scientific debate is centred around the diagnostic yield of these modalities and determining the optimal age and interval to start screening. However, due to the limited experience with screening, optimisation of MRI protocols is necessary to improve detection of pancreatic lesions in a screening setting.
Regular MRI protocols include T2-weighted sequences and T1-weighted series before and after administration of a contrast agent, often supplemented with magnetic resonance cholangiopancreaticography (MRCP). Normal pancreatic tissue is hyperintense on T1-weighted sequences relative to both inflammatory and malignant lesions, as well as cystic structures. In 2012 we added a T1-weighted turbo field echo (TFE) sequence to the protocol. This is a gradient recalled echo (GRE) sequence acquired after a 180 degree inversion recovery pulse, comparable to the magnetization prepared rapid acquisition gradient echo (MP-RAGE) sequence originally described in abdominal imaging for detecting liver lesions (11). By selecting an appropriate inversion time, contrast between healthy and abnormal pancreatic tissue can be increased, possibly facilitating the detection of small pancreatic lesions.
In this retrospective study, we assess the value of different MRI sequences in detection of PDAC in a high-risk surveillance cohort.
Methods
Prospective surveillance cohort
Since the start of the surveillance project in 2000, 218 individuals with the CDKN2A-p16-Leiden mutation have been enrolled (as of 04-01-2017) and are screened annually with MRI with optional EUS from the age of 45. A detailed description of the surveillance protocol has been published previously (7,8). An observation period of January 2012 until August 2017 was selected for its homogeneity in MRI protocols performed on the same 3T scanner. Images and pathology reports of subjects with a malignant pancreatic lesion detected during this observation period were collected. The study was approved by the Institutional Review Board of the Leiden University Medical Center (P00.107) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Oral or written consent was received from all patients.
MRI
MRI examinations were performed on a 3T system (Philips Ingenia; Philips Medical Systems, Best, The Netherlands) using a 32-channel torso coil. The standard MRI protocol for imaging of the pancreatic parenchyma included respiratory-triggered axial and coronal T2-weighted turbo spin echo (TSE) sequences, an axial breath-hold multiphase contrast-enhanced (MCE) T1-weighted 3D Dixon-based GRE sequence with water/fat separation before, at 20 seconds, 1 minute and 3 minutes after administration of a gadolinium-based contrast agent (Dotarem, Guerbet, France). The main pancreatic duct (MPD) was depicted with MRCP using a breath-hold thick slab T2-weighted radial TSE sequence.
The T1-TFE sequence was acquired with respiratory triggering. Before addition of the sequence to the screening protocol, the optimal inversion recovery time (TI) had been assessed in 3 healthy volunteers. Similar to the use of the sequence in liver imaging, the signal intensity (SI) of the spleen was used as an isointense standard for that of lesions in the pancreas. T1-TFE was acquired with inversion times of 800, 1,000, 1,200, 1,400 and 1,600 ms. SI of the pancreas and spleen were measured with a region of interest (ROI), the contrast ratio (CR) was calculated with the formula:
The lower inversion times provided the highest CR. An inversion time of 800 ms yielded the highest CR (average of −0.62) compared to an inversion time of 1,200 ms (CR average of −0.40). However, due to heterogeneity of the SI in the pancreas at lower inversion times we subjectively found an inversion time of 1,200 ms to provide the best balance between CR and image quality, which was also reflected by the larger standard deviation of SI at lower inversion times.
Diffusion weighted imaging (DWI) was added to the protocol in 2015. Apparent diffusion coefficient (ADC) maps were calculated from DWI obtained with single shot echo-planar imaging (EPI) using b-values of 0, 100, 500 and 1,000 s/mm2.
A comprehensive overview of MRI sequences and parameters is shown in Table 1.
Table 1
| Parameter | T1-TFE | T2-TSE | T1-MCE | DWI |
|---|---|---|---|---|
| Repetition time (ms) | 7 | 950 | 3.4 | 1,588 |
| Echo time (ms) | 2.3 | 80 | 1 | 64 |
| Echo train length | 96 | 70 | 2 | 29 |
| Flip angle (degrees) | 15 | 90 | 10 | 90 |
| Slice thickness (mm) | 4 | 4 | 3,5 | 6 |
| Spacing between slices (mm) | 4.4 | 4,4 | 1.75 | 6.6 |
| Number of phase encoding steps | 199 | 251 | 230 | 132 |
| Number of averages | 1 | 1 | 1 | 5 |
| Matrix size | 268×199 | 308×251 | 228×230 | 132×132 |
| Field of view (mm) | 400 | 400 | 400 | 400 |
| b-values | 0/10/500/999 | |||
| Inversion time (ms) | 1,200 | |||
| Acquisition | 2D | 2D | 3D | 2D |
| Fat suppression | No | No | Yes | Yes |
TFE, turbo field echo; TSE, turbo spin echo; MCE, multiphase contrast-enhanced; DWI, diffusion weighted imaging.
Image analysis
Three readers, two experienced abdominal radiologists (S.F. 5 and M.N.W. 20 years of experience) and one senior radiology resident (A.C.M.), independently analysed the images for the presence and location of lesions in the pancreas. First, they analysed the images of the examination on which the tumor was clinically reported. Next they re-evaluated the images of each previous examination, until no abnormalities were seen. For each sequence the readers documented whether a lesion was visible, reported the location and size of the lesion and reported any additional findings. All available previous MRI examinations were analysed in the same manner.
T1-TFE was considered positive when an area of reduced SI was seen in the pancreatic parenchyma, T2-TSE was considered positive in the case of focal increased SI. On T1-MCE, the presence of a lesion was reported before and after administration of contrast. The enhancement of PDAC is variable and consequently T1-MCE was considered positive when an area deviated in enhancement compared to the rest of the parenchyma. DWI was reported positive when high SI on high b-value DWI images corresponded spatially to low values on the ADC maps. The size of the lesions was based on measurements on T1-MCE or T1-TFE and an average of the readers’ measurements was calculated.
The size of the lesions on the preoperative MRI was compared with the size in the pathology report.
The contrast between lesions and normal pancreatic parenchyma was determined by measuring the SI on the preoperative MRI on T1-TFE, the unenhanced and the late-arterial phase of T1-MCE with a ROI. The CR was calculated with the following equation:
The average CR of lesions on T1-TFE was compared with the CR of the unenhanced and late arterial phase of the T1-MCE. A two tailed paired t-test was used to calculate P values.
Results
Between January 2012 and August 2017, 9 pancreatic cancers were detected in the mutation carriers at an average age of 60.3 years (s =7.8 years), 5 of whom were female. In 8 (89%) of these patients the tumor was resectable. The mean diameter of the lesions according to pathology reports was 13.4 mm (s =2.7 mm), the smallest tumor measuring 9 mm and the largest 18 mm.
In one subject a lesion was detected at the first screening round with a diameter of 24 mm measured on MRI. On microscopy, the diameter could not accurately be determined due to extensive fibrotic tissue surrounding the tumor.
Eight subjects underwent multiple examinations (average 7.9, s =2.2) during surveillance before an incident tumor was detected. One of the incident tumors was not resectable due to locally advanced disease.
The detectability of lesions for each of the 9 subjects on T1-TFE, T2-TSE, T1-MCE and DWI on the pre-operative MRI is summarized in Table 2, including retrospective findings during previous examinations, the size of the lesions based on MRI, as well as the diameter of the lesions according to pathology reports.
Table 2
| Subject | T1-TFE* | T2-TSE* | T1-MCE* | DWI* | Findings on previous examinations | Size at detection | Size on pathology reports |
|---|---|---|---|---|---|---|---|
| 1 | 3/3 | 1/3 | 1/3 | 2/3 | Lesion of 9 mm and dilated MPD detected by all readers 9 months prior on T1-TFE and one reader detecting a lesion of 5 mm on T1-TFE 25 months prior | 15 mm | 18 mm |
| 2 | 3/3 | 3/3 | 3/3 | NA | Lesion of 12 mm detected by all readers 12 months prior on T1-TFE | 17 mm | 15 mm |
| 3 | 1/3 | 1/3 | 2/3 | 3/3 | Dilated MPD detected by all readers 13 months prior and one reader detecting a possible lesion** of 5 mm on T1-TFE and T1-MCE | 10 mm | 15 mm |
| 4 | 3/3 | 3/3 | 3/3 | 1/3 | Lesion of 8 mm detected by one reader on T1-TFE and a possible of lesion** of 12 mm on T2-TSE by the second reader at 9 months prior, possible lesion** detected by the third reader on the arterial phase of the T1-MCE | 15 mm | 9 mm |
| 5 | 2/3 | 1/3 | 3/3 | NA | Lesion of 8 mm on T1-TFE detected by 2 readers and by one of these readers also on T1-MCE at 8 months prior | 14 mm | 15 mm |
| 6 | 3/3 | 3/3 | 3/3 | NA | Lesion of 7 mm on T1-TFE detected by two readers and detected on T2-TSE by one of these readers at 12 months prior | 10 mm | 9 mm |
| 7 | 3/3 | 3/3 | 3/3 | 3/3 | None | 14 mm | 13 mm |
| 8 | 3/3 | 3/3 | 3/3 | NA | None | 19 mm | No resection performed |
| 9 | 3/3 | 3/3 | 3/3 | NA | No previous examinations | 24 mm | Could not be accurately determined |
*, these results are shown as a function in which the numerator corresponds to the number of readers reporting the sequence positive for a lesion. **, the term ‘possible lesion’ means the reader was unsure whether the finding represented a true lesion. PDAC, pancreatic ductal adenocarcinoma; TFE, turbo field echo; TSE, turbo spin echo; MCE, multiphase contrast-enhanced; DWI, diffusion weighted imaging; DWI, diffusion weighted imaging.
Images of a subject with a tumor in the pancreatic tail, including a T1-TFE image, are shown in Figure 1.
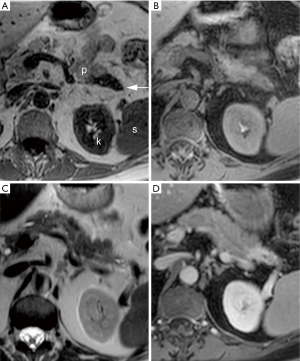
Both T1-TFE and T1-MCE were reported positive for a lesion by all three readers in 7/9 subjects (78%) and by at least two readers in 8/9 (89%) subjects. T2-TSE was reported positive by all three readers in 6/9 subjects (67%) and only reported positive by one reader in the other 3 subjects. Increased enhancement relative to the pancreas on the delayed phase of T1-MCE was present in 7 PDAC. DWI was available in 4 patients with PDAC in this cohort and was reported positive in 2/4 subjects by three readers and by respectively two readers and a single reader in the other 2 subjects.
Dilatation of the pancreatic duct was present in 3 subjects, 2 separate subjects demonstrated sole dilation of the biliary tract. No cystic lesions were associated with the PDAC.
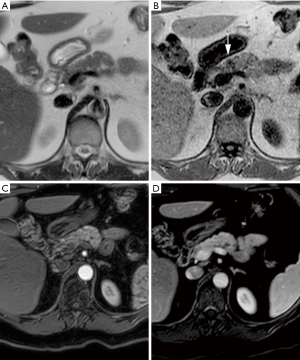
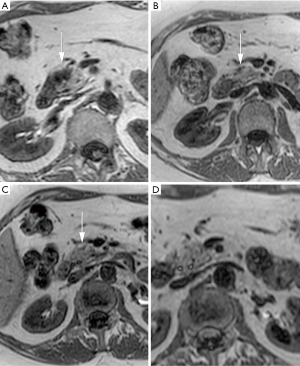
Through retrospective analysis, lesions and other findings were reported in the previous examinations of 6/8 subjects with an incident tumor, as summarized in Table 2. In two of these cases a lesion was reported by all readers only on the T1-TFE sequence of the previous examination, demonstrated in Figures 2 and 3. In the other 4 subjects a lesion or indirect signs of a lesion, such as change in MPD diameter, were reported by either one or two readers.
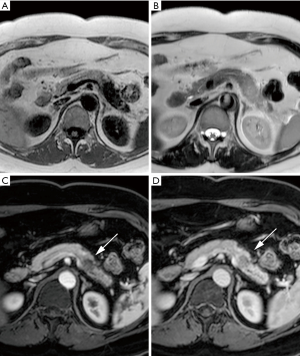
A prevalent tumor of 24 mm in the tail of the pancreas resulted in extensive inflammation in the upstream portion of the tail visible on the T2 and contrast-enhanced images. On T1-TFE, the entire upstream portion of the tail showed decreased SI as shown in Figure 4.
The CR was calculated in 8 lesions which were visible on both T1-TFE and T1-MCE. The average CR of lesions was −0.63 (s=0.09) on T1-TFE, −0.36 (s =0.11) on unenhanced T1-MCE and −0.41 (s =0.12) on the late-arterial phase T1-MCE. The CR of lesions on T1-TFE was 75% greater compared to unenhanced T1-MCE (P=0.0001) and the CR of lesions on T1-TFE was also 58% greater compared to the late-arterial phase T1-MCE (P=0.0004).
During the observation period two lesions were detected on MRI and confirmed at EUS, but proved to be benign after resection. The first case was a 12 mm mass with calcifications in the uncinate process which was visible on all MRI sequences, pathology showed an intraductal mucinous neoplasm with low grade dysplasia. The second case was a 7 mm lesion in the pancreatic tail visible on T1-TFE and T1-MCE, a concurrent mass in the stomach was also found on MRI. Resection of the pancreatic tail and gastric lesion was performed. Pathology demonstrated a low grade pancreatic intraepithelial neoplasm and a gastrointestinal stromal tumor (GIST) of the stomach.
Discussion
Pancreatic cancer remains one of the most lethal malignancies with a five-year survival rate of 8% for all stages. For localized disease five-year survival rates increase to 32% (1). The size of PDAC at diagnosis is an important prognostic factor, with several studies reporting a considerable increase in resectability and survival for lesions of 20 mm or smaller (12-14). Lymph node involvement also increases with increasing tumor size (13). However, size is not an independent prognostic factor as a linear correlation between tumor size and survival has only been demonstrated for localised disease (15). It is clear that early detection is of paramount importance for a favourable outcome, and screening should aim to detect lesions at the smallest size possible before irresectable neural plexus invasion, vascular invasion or either lymphatic or distant metastases have occurred.
Eight out of 9 (89%) of the PDAC detected during the observation period of this study were resectable. This resectability rate is much higher than the rate reported in sporadic pancreatic cancer.
During the observation period, a pancreatic lesion was detected in two mutation carriers on both MRI and EUS which turned out to be benign after resection. This is an illustration of the dilemmas that may occur during a surveillance program for pancreatic cancer.
The T1-TFE sequence performed comparable to T1-MCE in detecting lesions on the pre-operative MRI. In one patient, the tumor was located at the hepatopancreatic ampulla where no surrounding healthy pancreatic tissue was present and the T1-TFE was reported negative by two readers. However, although causing dilatation of the MPD, the lesion itself was equally difficult to detect on other sequences.
On T1-TFE the pancreas has a high SI, attributable to the high aqueous protein content in the pancreatic acini as well as the abundance of endoplasmic reticulum and various paramagnetic ions in the protein producing acinar cells (16). Solid pancreatic neoplasms and cystic lesions do not possess this molecular make-up and thus have longer T1-relaxation times, represented by a decreased SI on T1-weighted images. Similar changes during pancreatitis likewise lead to a decreased SI (17), which also can be induced by a PDAC causing up-stream obstruction of the pancreatic duct. These imaging characteristics are also true for other T1-weighted sequences such as the unenhanced phase of the T1-MCE, but are amplified by the inversion recovery pulse of the T1-TFE sequence, increasing contrast between healthy and abnormal pancreatic tissue. Indeed, the CR of lesions was significantly greater on T1-TFE compared with both the unenhanced and the late-arterial phase of T1-MCE. The formula used for calculation of the CR was chosen instead of a contrast-to-noise ratio because it takes into account the background SI of the pancreas in relation to the conspicuity of the lesion, as elaborated by Downs (18).
PDAC is one of the most stroma-rich malignancies (19), leading to a poorly enhancing lesion visible on the arterial phase of dynamic post-contrast imaging. In smaller tumors this appearance is often not present. The extensive extra-cellular space of the lesions causes retention of gadolinium, which is the reason for increased enhancement on the delayed phase of T1-MCE. Indeed this late enhancement pattern was present in 7 of the 9 PDAC and is a valuable sign for the detection of PDAC. However, late enhancement is not specific for PDAC as other causes of fibrosis can demonstrate this likewise. For these reasons, every phase of the post-contrast images should be scrutinized.
Some pancreatic cancers demonstrate hyperintensity on T2-weighted images, but often are inconspicuous on these sequences. Nonetheless, T2-weighted sequences produce high quality anatomical information and provide a detailed view of the MPD, of which changes can be the first and only sign of developing PDAC as we demonstrated previously (20). No cystic lesions were associated with the PDAC detected in the observation period, suggesting lesions such as intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasms are not common precursor lesions in the development of PDAC in this population.
In the observation period only 4 PDACs have been detected after the addition of DWI. Higher b-value images often contained considerable imaging artefacts, reducing interpretability. Other researchers have found the mean ADC value of pancreatic cancer to be significantly lower compared to the normal pancreas, and also lower than tissue affected by pancreatitis (21,22).
In 6 of 8 incident PDAC, direct or indirect signs of a tumor were retrospectively detected on previous examinations. This is a well-known consequence of screening, as knowledge of the developing tumor enables radiologists to detect subtle findings that were overseen at the initial assessment. This effect has been demonstrated in pancreatic cancer previously (23). T1-TFE was the most sensitive sequence in detecting abnormalities on the previous examinations. In all 6 cases abnormalities were reported on T1-TFE by at least one reader. In two of these 6 cases, a lesion was identified only on the T1-TFE images by all readers, suggesting T1-TFE could be able to detect PDAC before it is visible on other MRI sequences. Data acquired through retrospective analysis cannot be used to determine diagnostic performance due to outcome bias. However, knowledge of these imaging findings may enable experienced readers to detect these developing tumors at a smaller size.
There are several limitations to this study. First, although this is one of the largest screening cohorts of hereditary pancreatic cancer, still only a limited number of PDACs have been detected through screening. Second, in a retrospective analysis there is potential for bias. However, study subjects and data were included in a prospective manner after which MRI features were analysed retrospectively. Third, this retrospective analysis lacks a negative control group to identify possible false positive results. Nonetheless, individual sequences have not been responsible for wrongful surgery in clinical practise, as findings were verified with a second imaging modality (EUS or CT) and confirmation of malignancy was obtained through biopsy whenever possible. As stated, two benign precursor lesions were resected during the observation period. On the other hand, the findings of early malignant changes in the pancreas that were detected by the readers on the previous examinations lack histologic proof. Lastly, considering the later addition of DWI and artefacts we observed, further optimization of the sequence is necessary before conclusions can be drawn on the value of DWI in screening.
Annual MRI surveillance in asymptomatic individuals at high risk of developing PDAC results in detection of tumors at a potentially resectable stage. A T1-weighted sequence with inversion recovery increases contrast between normal and abnormal pancreatic tissue and may therefore be of additional value in screening of a high risk population. Knowledge of imaging features and recognition of subtle changes in the pancreas on various MRI sequences may improve detection of early pancreatic cancer.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apc-19-50
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-19-50). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of the Leiden University Medical Center (P00.107) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Oral or written consent was received from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. Ca Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Bartsch DK, Slater EP, Carrato A, et al. Refinement of screening for familial pancreatic cancer. Gut 2016;65:1314-21. [Crossref] [PubMed]
- Grover S, Jajoo K. Screening for Pancreatic Cancer in High-risk Populations. Gastroenterol Clin North Am 2016;45:117-27. [Crossref] [PubMed]
- Torphy RJ, Schulick RD. Screening of Patients at Risk for Familial Pancreatic Cancer: What Is Beneficial? Surg Clin North Am 2018;98:25-35. [Crossref] [PubMed]
- Vasen HF, Gruis NA, Frants RR, et al. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden). Int J Cancer 2000;87:809-11. [Crossref] [PubMed]
- Vasen HF, Wasser M, van Mil A, et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology 2011;140:850-6. [Crossref] [PubMed]
- Vasen H, Ibrahim I, Ponce CG, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol 2016;34:2010-9. [Crossref] [PubMed]
- DaVee T, Coronel E, Papafragkakis C, et al. Pancreatic cancer screening in high-risk individuals with germline genetic mutations. Gastrointest Endosc 2018;87:1443-50. [Crossref] [PubMed]
- Canto MI, Almario JA, Schulick RD, et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018;155:740-751.e2. [Crossref] [PubMed]
- Harinck F, Konings IC, Kluijt I, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016;65:1505-13. [Crossref] [PubMed]
- de Lange EE, Mugler JP 3rd, Bertolina JA, et al. Magnetization prepared rapid gradient-echo (MP-RAGE) MR imaging of the liver: comparison with spin-echo imaging. Magn Reson Imaging 1991;9:469-76. [Crossref] [PubMed]
- Agarwal B, Correa AM, Ho L. Survival in pancreatic carcinoma based on tumor size. Pancreas 2008;36:e15-e20. [Crossref] [PubMed]
- Petermann D, Demartines N, Schäfer M. Is tumour size an underestimated feature in the current TNM system for malignancies of the pancreatic head? HPB (Oxford) 2013;15:872-81. [Crossref] [PubMed]
- Hur C, Tramontano AC, Dowling EC, et al. Early Pancreatic Ductal Adenocarcinoma Survival Is Dependent on Size: Positive Implications for Future Targeted Screening. Pancreas 2016;45:1062-6. [Crossref] [PubMed]
- Ansari D, Bauden M, Bergström S, et al. Relationship between tumour size and outcome in pancreatic ductal adenocarcinoma. Br J Surg 2017;104:600-7. [Crossref] [PubMed]
- Pamuklar E, Semelka RC. MR imaging of the pancreas. Magn Reson Imaging Clin N Am 2005;13:313-30. [Crossref] [PubMed]
- Sica GT, Miller FH, Rodriguez G, et al. Magnetic resonance imaging in patients with pancreatitis: evaluation of signal intensity and enhancement changes. J Magn Reson Imaging 2002;15:275-84. [Crossref] [PubMed]
- Downs RK, Bashir MH, Ng CK, et al. Quantitative contrast ratio comparison between T1 (TSE at 1.5T, FLAIR at 3T), magnetization prepared rapid gradient echo and subtraction imaging at 1.5T and 3T. Quant Imaging Med Surg 2013;3:141-6. [PubMed]
- Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res 2012;18:4266-76. [Crossref] [PubMed]
- Vasen HFA, Boekestijn B, Ibrahim IS, et al. Dilatation of the main pancreatic duct as first manifestation of small pancreatic ductal adenocarcinomas detected in a hereditary pancreatic cancer surveillance program. HPB (Oxford) 2019;21:1371-5. [Crossref] [PubMed]
- De Robertis R, Tinazzi Martini P, Demozzi E, et al. Diffusion-weighted imaging of pancreatic cancer. World J Radiol 2015;7:319-28. [Crossref] [PubMed]
- Barral M, Soyer P, Ben Hassen W, et al. Diffusion-weighted MR imaging of the normal pancreas: reproducibility and variations of apparent diffusion coefficient measurement at 1.5- and 3.0-Tesla. Diagn Interv Imaging 2013;94:418-27. [Crossref] [PubMed]
- Gangi S, Fletcher JG, Nathan MA, et al. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol 2004;182:897-903. [Crossref] [PubMed]
Cite this article as: Boekestijn B, Feshtali S, Meijer AC, Ibrahim IS, Farina-Sarasqueta A, Inderson A, Bonsing BA, Webb AG, Vasen HF, Wasser MN. MRI screening in hereditary pancreatic cancer: value of various sequences in the detection of early pancreatic cancer. Ann Pancreat Cancer 2020;3:16.


