Association of germline ATM mutations and survival in pancreatic cancer
Introduction
Pancreatic cancer is a lethal disease with 5-year overall survival (OS) of only 9% (1). There is no current screening guideline to detect asymptomatic early-stage pancreatic cancer and a majority of patients present with locally advanced or metastatic disease. Additionally, defining biological features include activating mutations KRAS oncogene in over 90% of patients and loss or mutations of tumor suppressor genes, likely contributing to the aggressive phenotype of pancreatic cancer (2). Next-generation sequencing (NGS) of pancreatic tumors and germline multigene panel testing of pancreatic cancer patients has revealed genes that predispose patients to pancreatic cancer. Mutations in CDKN2A, TP53, BRCA1, BRCA2, ATM, PALB2, MLH1, among others, are significantly associated with pancreatic cancer risk (3-7), and approximately 4% to 10% of patients with pancreatic adenocarcinoma have pathogenic germline alterations (8). A recent study from 2020 showed up to 19% of pancreatic cancer patients had identified deleterious germline mutations (9).
As a biomarker for treatment selection, certain germline mutations have actionable therapeutic implications. Approximately 3% to 7% of pancreatic ductal adenocarcinomas (PDAC) harbor loss of function mutation in BRCA1, BRCA2, or both (BRCA) genes. In the phase 3 POLO trial, patients who harbor germline BRCA mutations have improved progression-free survival (PFS) from poly-ADP (adenosine diphosphate)-ribose polymerase inhibitor (PARPi) olaparib in the maintenance setting (7.4 vs. 3.8 months) (10). Patients with microsatellite instability (MSI) or mismatch repair deficiency may benefit from immunotherapy (11). As pancreatic adenocarcinoma has entered the era of precision medicine, germline testing in addition to NGS for somatic gene mutation testing is now routinely tested for all patients, even in patients without significant family history of cancer (12). In addition to potential therapeutic and prognostic information, germline testing allows family members to possibly benefit from cancer screening and strategies to prevent cancer (13,14).
Ataxia telangiectasia mutated (ATM) gene, located on 11q22-23, encodes a 3056 amino acid PI3K-related protein consisting of a: (I) N-terminal TAN [telomere length maintenance and DNA damage repair (DDR)] domain (residues 7-165); (II) FAT domain (residues 2097-2488); (III) C-terminal kinase domain with genomic similarity to PI3K (residues 2714-2961); (IV) FATC domain (residues 3055-3205) (15,16). ATM gene, in its autosomal recessive form, is associated with ataxia-telangiectasia syndrome, a clinically heterogeneous syndrome characterized by progressive cerebellar ataxia, telangiectasia, and susceptibility to hematological malignancies. ATM has a critical role in the recognition and response to DNA double-strand breaks (DSBs) to maintain the integrity of the genome. When activated by DNA DSBs and/or changes in chromatic structure, ATM signals the cell to slow the passage through cell-cycle checkpoints to facilitate DNA repair (16-18). Therefore, mutated ATM will lead to the accumulation of mutations over time which ultimately increases the risk of malignancy.
ATM mutations confer a greater risk of multiple types of solid tumors including breast cancer and ovarian cancer (19,20). In a recent case-control analysis with 3,030 pancreatic cancer patients, germline ATM mutations were observed in a significantly higher number of cases than controls (2.3% vs. 0.37%) (3). Additionally, pancreatic cancer patients with germline mutations in DDR genes that are pathogenic or likely pathogenic (LP) had a superior OS than non-carriers (median OS 34.4 vs. 19.1 months, respectively). However, the analysis comprised of resected patients and only 4 germline ATM mutations (21). A recent study in deleterious germline mutated metastatic pancreatic cancer patients showed a survival benefit in patients with DDR gene mutations treated with FOLFIRINOX, however, it only included 3 germline ATM patients (9).
There is mounting evidence that germline mutations in DDR genes confer survival benefit in pancreatic cancer (9,10,21), yet the influence of germline ATM mutations on OS in pancreatic cancer has not been established. Such studies may provide insight into pancreatic cancer prognosis, and may have implications for selection of therapy and personalized clinical management. Thus, a better understanding of the role of germline ATM mutations in pancreatic cancer is needed. The objective of this study was to evaluate pancreatic cancer patients with germline and somatic tumor testing and to compare PFS and OS to standard chemotherapy in those: (I) with germline ATM mutations; (II) with somatic ATM mutations; and (III) without ATM mutations.
Methods
Study population
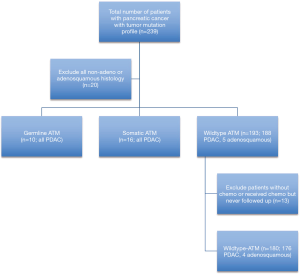
This study included 239 patients who were seen at a single institution between 2007 and 2019 with biopsy confirmed pancreatic cancer. Cases were identified retrospectively and all patients had consented for DNA banking for research purposes. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board (IRB Pro00054363). All 239 patients provided informed consent for NGS testing (Perthera, Foundation One, Guardant360, Caris Life Sciences) of their tumors by March 20th, 2020. Neuroendocrine differentiation, pancreatic acinar cell carcinoma, and pancreatoblastoma on biopsy were excluded given the low prevalence in our collection (n=20) with 219 patients remaining (Figure 1). Test requisitions were completed by the ordering clinicians. Information included personal history of cancer, age at diagnosis, cancer pathology, genetic testing, tumor molecular profiling, and family history of cancer. Information was extracted from available clinical records including clinic notes, pathology reports, and genetic tests. Lost to follow up was defined by any patient without available clinical records within the prior 12 months.
ATM gene mutation screening
In total, 67 of the 219 patients had multigene germline testing (Invitae, Myriad MyRisk, FoundationOne Liquid, and Ambry Genetics PancNext). Germline testing was performed using peripheral blood samples except for two patients where saliva was used. There are no substantial differences in methodologies or potential consequences between saliva and blood. Germline testing was performed in patients who had a high clinical suspicion for hereditary cancer risk based on age at diagnosis, personal history of cancer, and family history of cancer, however, no set algorithm was used to guide testing. All patients provided informed consent.
Assessments
PFS was calculated from the date of first-line chemotherapy until the date of objective radiologic disease progression, as defined according to RESIST, version 1.1, or death in patients without disease progression. OS was calculated from the date of first-line chemotherapy until date of death. Data was collected on April 10th, 2020 and patients were analyzed based on this data cut-off date.
Statistical analysis
Pancreatic cancer patients were assessed by comparisons using cox-regression models with adjustment for age at diagnosis, treatment type, gender, stage of disease, and resection status. Hazard ratios (HR) and corresponding 95% confidence intervals were estimated. All tests are two-sided and are considered statistically significant if P values were less than 0.05.
Results
Study population characteristics
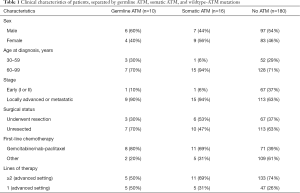
Next-generation sequencing of somatic gene testing was complete in 239 pancreatic cancer patients; 219 (91.6%) of these patients had PDAC (n=214) or adenosquamous carcinoma (n=5). Sixteen of the 239 (6.7%) patients had somatic ATM mutation. There were 67/239 (28.0%) patients who had additional germline genetic testing with 10 (14.9%) confirmed patients with germline ATM mutations. Fifteen of the 67 patients tested for germline mutations had non-ATM germline mutations (Table S1). Thirteen of 219 patients with adenosquamous or PDAC were excluded from analysis as they never received chemotherapy, or received chemotherapy and never followed up with our institution and medical records were not obtained. All 13 of these patients had wildtype ATM, thus 180 wildtype ATM patients remained for analysis (Figure 1). The clinical characteristics including categorical variables such as gender, history of resection, stage are reported in given frequencies and percentages and separated by ATM mutation status (Table 1). All patients with somatic or germline ATM mutations had pathologically confirmed adenocarcinoma. The median age of diagnosis of wildtype ATM, somatic ATM, and germline ATM mutated patients were 65.3 (range, 38.3 to 96.1), 71.9 (range, 56.7 to 86.4), and 67.8 (range, 48.8 to 78.2) years, respectively. Three of the 10 patients with germline ATM mutations had a secondary cancer (https://cdn.amegroups.cn/static/public/apc-20-38-1.xlsx).
Full table
ATM mutation prevalence in pancreatic cancer
Germline ATM mutations were detected in 14.9% of patients tested (10 out of 67). Six of the 10 germline ATM patients had additional germline mutations, of which 5 of the 6 (83.3%) mutations were variants of unknown significance (VUS; https://cdn.amegroups.cn/static/public/apc-20-38-1.xlsx). Four of the 10 germline ATM patients were VUS. One patient had a germline CHEK2 c.1427C>T mutation that has been shown to be both a VUS and possibly pathogenic (22). Somatic ATM mutations were detected in 6.7% of patients. Of the 16 patients with somatic ATM mutations, only 3 were tested for germline mutations. Characteristics of pancreatic cancer patients with germline or somatic ATM mutation are shown in https://cdn.amegroups.cn/static/public/apc-20-38-1.xlsx.
Impact of ATM on OS
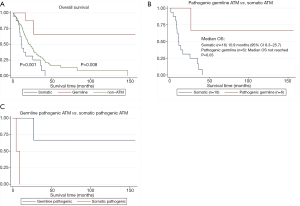
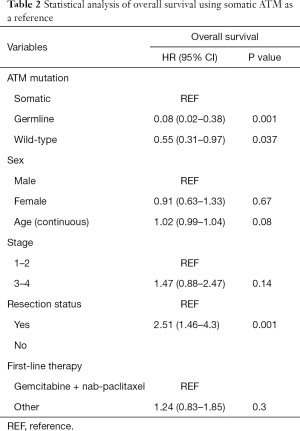
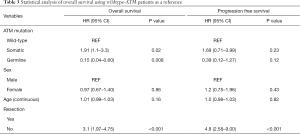
Among those with somatic ATM mutations, 15 of the 16 patients had died. Among those with germline ATM mutation, 2 of the 10 patients had died. Eight out of 9 germline ATM mutated patients were treated with gemcitabine and nab-paclitaxel (one had unknown chemotherapy) compared to 12 out of 16 somatic patients. The median OS was significantly longer (Figure 2A) in the germline ATM group compared to somatic ATM (Table 2; 21.5 vs. 11.3 months; HR 0.08, 95% CI, 0.02–0.38; P=0.001) or wildtype-ATM group (Table 3; 14.2 months; HR 0.15, 95% CI, 0.04–0.6; P=0.008). When grouping the germline and wildtype-ATM groups together, this combined cohort had significantly longer OS than somatic ATM patients (P=0.018; Figure S1). Additionally, the somatic ATM group had significantly worse survival benefit compared to the wildtype-ATM group (HR 1.91, 95% CI, 1.1–3.3; P=0.02) (Table 3). When stratifying based on pathogenicity of germline ATM, pathogenic germline ATM (n=6) was associated with a significant increase in OS versus somatic ATM (P=0.03; Figure 2B). When stratifying based on pathogenicity of somatic ATM, pathogenic somatic ATM (n=2) had non-significant survival benefit (Figure 2C).
Full table
Full table
When using cox-regression models to analyze for resection status, patients without tumor resection had worse OS than those with resection (HR 2.51, 95% CI, 1.46–4.3; P=0.001). However, patients with local disease (stage 1–2) did not show significant survival benefit over advanced stage (stage 3–4) patients (HR 1.47, 0.88–2.47; P=0.14). There was no statistically significant difference in survival when treating with a first-line chemotherapy regimen containing gemcitabine and nab-paclitaxel (P=0.3).
Impact of ATM on PFS
Among those with somatic ATM mutations, 10 of the 16 patients had progression on first-line treatment. Among those with germline ATM mutation, 6 of the 10 patients had progression on first-line treatment. The median PFS was longer in the germline ATM group than in the somatic ATM (13.6 vs. 8.0 months; HR 0.41, 95% CI, 0.14–1.16; P=0.09) or wildtype-ATM group (9.2 months; HR 0.39, 95% CI, 0.12–1.27; P=0.12), however, neither were statistically significant (Figure 3A). When stratifying based on pathogenicity of germline ATM, pathogenic germline ATM (n=6) showed a non-significant trend towards improved PFS (P=0.07; Figure 3B). Similarly, when comparing pathogenic germline and somatic ATM (n=2), germline ATM showed a non-significant trend towards improved PFS (Figure 3C).
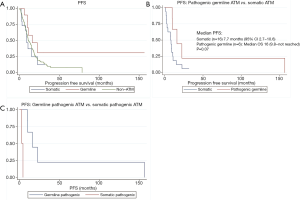
There was no significant difference in PFS by resection status (P=0.08) or staging (P=0.37; Table 4). Analysis of the groups based on first-line treatment of gemcitabine and nab-paclitaxel did not show significant improvement in PFS in the germline ATM patients.
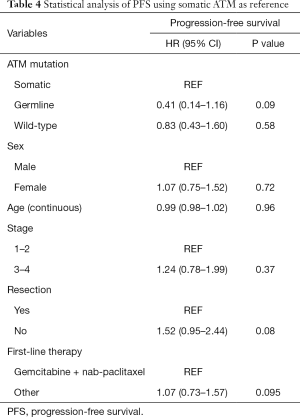
Full table
Discussion
Germline DDR mutations in pancreatic cancer are associated with better OS in pancreatic cancer (9,21). We report on 219 pancreatic cancer patients from a cohort assembled by a molecular profiling of tumor with pathology information and hereditary cancer genetic testing. There were 6 of 10 patients with pathogenic or LP germline ATM mutations and 2 of 16 patients with pathogenic somatic ATM mutations. The results showed that pathogenic or combined germline ATM mutations are statistically associated with improved OS in pancreatic cancer compared to wildtype or somatically-mutated ATM pancreatic cancer. Although PFS in germline ATM mutations was longer, it was not statistically significant compared to wildtype or somatic-ATM mutated patients. Given the function of ATM in the repair of DNA DSBs, this study suggests that pathogenic germline ATM mutations renders tumor cells more responsive to overall treatment allowing for greater survival benefit, and/or that the biology of the tumor is less aggressive.
In most cases, ATM mutations give rise to truncated ATM protein through nonsense mutations, however, some mutations can be missense or in-frame deletions producing inactive ATM (16). Those with a high incidence of cancer in Ataxia-Telangiectasias had a high frequency of missense mutation (23). Missense mutations can cause a dominant-negative effect of ATM function leading to reduction in ATM function that is greater than in carriers of truncation mutation (24). Based on allelic frequency, carriers of missense mutation may have both functional and inactive ATM in different ratios (16). Thus, recognizing that different allele frequencies can lead to different phenotypes is critical. Importantly, germline mutations in the clinic would have >50% allele frequency of any given mutation. This may be why germline ATM mutations are significantly associated with better outcomes compared to somatic ATM mutations.
Differentiating somatic driving events of tumorigenesis from passenger mutations is a major genomic challenge. There is evidence of clonal evolution within metastases of pancreatic cancer, and mutated ATM could be a byproduct of this evolution as there is considerable heterogeneity among cells that initiate metastasis (25). Therefore, somatic ATM alterations could possibly be passenger or non-oncogenic driver mutations whether they are labeled as pathogenic or VUS. In our somatic ATM mutated cohort, 2 of 16 patients had pathogenic ATM mutations and lived for 4.3 and 8.9 months, respectively, which is worse survival than the germline cohort albeit a small sample size. Without serial NGS, it is unknown when along the clinical disease course that the somatic ATM mutations occurred; perhaps later in their disease course leading to worse outcomes. Additionally, we cannot exclude the possibility that germline ATM mutations labeled as VUS may, in fact, result in real differences in clinical outcome. The oncogenicity of ATM mutations must be further evaluated to answer this question. There are many other signaling pathways which are hallmarks of pancreatic cancer, including apoptosis, angiogenesis, and TGF-β signaling, and somatic NGS profiling of patients without germline ATM mutation showed evidence of such alterations (Figure S2) (26).
PFS in germline ATM ranged from 6 to 156 months (https://cdn.amegroups.cn/static/public/apc-20-38-1.xlsx). The lower end of the spectrum suggests that mutated ATM alone was not sufficient to confer a durable response to chemotherapy and may not be solely responsible for the maintenance of the integrity of the genome. In fact, up to 90% of DNA DSBs are repaired by non-homologous end-joining repair through an ATM-independent mechanism (17). Since 70% of the germline ATM mutated patients had other concomitant germline mutations, it is possible that other germline or somatic mutations contribute to differential responses to treatment, particularly if those genomic alterations are involved in the DDR pathway. For example, patient 3 and 9 had germline ATM and BRCA1 mutations; BRCA1 interacts downstream with ATM to assist in cell-cycle control and maintaining genomic integrity (17). However, the mechanisms of response to a patient with both germline ATM and BRCA1 mutations has not been elucidated. There is evidence that have showed that tumoral ATM loss and normal p53 is associated with worse survival than in patients with mutated p53, suggesting that commonly mutated cancer genes can affect survival in patients with ATM mutations (27).
ATM mutations confer sensitivity and synthetic lethality to PARP inhibition in preclinical models (28-30). Similarly, BRCA1 and BRCA2 are tumor suppressor genes involved in DNA repair, and germline mutations in these genes confer sensitivity to PARP inhibitors leading to significant clinical benefit in pancreatic cancer in the 2019 POLO trial (10). In trials of metastatic castration-resistant prostate cancer, patients with DDR mutations including ATM aberrations had statistically better PFS with olaparib treatment. Although olaparib showed an insignificant survival benefit, this was likely due to high percentage of patients crossing over to olaparib from the control group after progressing on standard therapy (31,32). Response to olaparib occurred even in patients with monoallelic ATM mutations, suggesting that despite the presence of a 2nd wildtype allele, germline ATM mutations may have a dominant negative effect on the tumor (31). Our study highlights the importance of precision medicine in pancreatic cancer, further cemented by the national Know Your Tumor (KYT) registry trial for pancreatic cancer patients. In the KYT retrospective analysis, 26% (282/1,082) of pancreatic cancer patients had actionable molecular alterations that could be exploited therapeutically. In comparing pancreatic cancer patients who received molecularly matched therapies (n=46) to those that received non-molecularly matched therapies (n=143), OS was significantly improved (2.4 vs. 1.5 years) (33).
Germline ATM mutations may represent a prognostic marker for patients diagnosed with metastatic pancreatic cancer. Furthermore, exploiting this genetic defect therapeutically with combinations based on synthetic lethality could likely lead to better clinical outcomes, as set in precedent with the POLO trial. Additional research into the molecular underpinnings for pancreatic cancer patients with germline ATM mutations is warranted. Functional studies of these various ATM mutations will likely reveal a bigger role for ATM in the susceptibility of pancreatic cancer.
Our study was limited by small sample size and the numbers at risk are small. Additionally, not every patient was tested for germline mutations as the germline testing was not routinely performed in the past and was done based on clinician’s suspicion of hereditary predisposition, thus we cannot prove that everyone who did not have germline testing truly did not have a germline mutation. However, the clinical suspicion is low for these patients. Another limitation is that most patients were metastatic, therefore, cannot necessarily be applied to those with early stage disease. However, there is evidence of survival benefit in patients with germline mutations in resected pancreatic cancer patients compared to non-carriers (21). Additionally, most patients present with metastatic disease as there is no standard screening for pancreatic cancer. Lastly, due to the retrospective nature of this study, a vast majority of patients were deceased, introducing retrospective bias. However, we suggest that the validity of the survival benefit can be corroborated based on prior studies of germline DDR mutations and their association with OS.
Conclusions
Our findings suggest that germline ATM mutations may be associated with survival benefit compared to wildtype ATM or somatically-mutated ATM in metastatic pancreatic adenocarcinoma. Pancreatic cancer has fully entered the era of precision medicine, and this study shows that germline ATM may be of prognostic significance, and may possibly be exploited therapeutically with targeted therapies such as PARP inhibitors. Ongoing research is needed to understand the significance of ATM mutations with regard to functionality of the mutation, clinical significance, and actionability.
Acknowledgments
Work was supported by the Samuel Oschin Cancer Center at Cedars Sinai Medical Center.
Funding: None.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apc-20-38
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-20-38). JL reports that he is on the advisory board for Invitae. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Institutional Review Board (IRB Pro00054363). Patients had given their informed consent for genetic analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:2140-1. [Crossref] [PubMed]
- Hu C, Hart SN, Polley EC, et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018;319:2401-9. [Crossref] [PubMed]
- Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science 2009;324:217. [Crossref] [PubMed]
- Zhen DB, Rabe KG, Gallinger S, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med 2015;17:569-77. [Crossref] [PubMed]
- Roberts NJ, Norris AL, Petersen GM, et al. Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Cancer Discov 2016;6:166-75. [Crossref] [PubMed]
- Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2012;2:41-6. [Crossref] [PubMed]
- Rainone M, Singh I, Salo-Mullen EE, et al. An Emerging Paradigm for Germline Testing in Pancreatic Ductal Adenocarcinoma and Immediate Implications for Clinical Practice: A Review. JAMA Oncol 2020;6:764-71. [Crossref] [PubMed]
- Goldstein JB, Zhao L, Wang X, et al. Germline DNA Sequencing Reveals Novel Mutations Predictive of Overall Survival in a Cohort of Patients with Pancreatic Cancer. Clin Cancer Res 2020;26:1385-94. [Crossref] [PubMed]
- Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline. N Engl J Med 2019;381:317-27. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Shindo K, Yu J, Suenaga M, et al. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J Clin Oncol 2017;35:3382-90. [Crossref] [PubMed]
- Vasen H, Ibrahim I, Ponce CG, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol 2016;34:2010-9. [Crossref] [PubMed]
- Abe T, Blackford AL, Tamura K, et al. Deleterious Germline Mutations Are a Risk Factor for Neoplastic Progression Among High-Risk Individuals Undergoing Pancreatic Surveillance. J Clin Oncol 2019;37:1070-80. [Crossref] [PubMed]
- Jette NR, Kumar M, Radhamani S, et al. ATM-Deficient Cancers Provide New Opportunities for Precision Oncology. Cancers (Basel) 2020;12:687. [Crossref] [PubMed]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 2003;3:155-68. [Crossref] [PubMed]
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 2008;9:759-69. [Crossref] [PubMed]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003;421:499-506. [Crossref] [PubMed]
- Lu HM, Li S, Black MH, et al. Association of Breast and Ovarian Cancers With Predisposition Genes Identified by Large-Scale Sequencing. JAMA Oncol 2019;5:51-7. [Crossref] [PubMed]
- Hu C, Polley EC, Yadav S, et al. The contribution of germline predisposition gene mutations to clinical subtypes of invasive breast cancer from a clinical genetic testing cohort. J Natl Cancer Inst 2020;112:1231-41. [Crossref] [PubMed]
- Yurgelun MB, Chittenden AB, Morales-Oyarvide V, et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med 2019;21:213-23. [Crossref] [PubMed]
- Schubert S, van Luttikhuizen JL, Auber B, et al. The identification of pathogenic variants in BRCA1/2 negative, high risk, hereditary breast and/or ovarian cancer patients: High frequency of FANCM pathogenic variants. Int J Cancer 2019;144:2683-94. [Crossref] [PubMed]
- Stankovic T, Kidd AM, Sutcliffe A, et al. ATM mutations and phenotypes in ataxia-telangiectasia families in the British Isles: expression of mutant ATM and the risk of leukemia, lymphoma, and breast cancer. Am J Hum Genet 1998;62:334-45. [Crossref] [PubMed]
- Gatti RA, Tward A, Concannon P. Cancer risk in ATM heterozygotes: a model of phenotypic and mechanistic differences between missense and truncating mutations. Mol Genet Metab 1999;68:419-23. [Crossref] [PubMed]
- Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010;467:1109-13. [Crossref] [PubMed]
- Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491:399-405. [Crossref] [PubMed]
- Kim H, Saka B, Knight S, et al. Having pancreatic cancer with tumoral loss of ATM and normal TP53 protein expression is associated with a poorer prognosis. Clin Cancer Res 2014;20:1865-72. [Crossref] [PubMed]
- McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006;66:8109-15. [Crossref] [PubMed]
- Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res 2012;72:5588-99. [Crossref] [PubMed]
- Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci U S A 2011;108:3406-11. [Crossref] [PubMed]
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015;373:1697-708. [Crossref] [PubMed]
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020;382:2091-102. [Crossref] [PubMed]
- Pishvaian MJ, Blais EM, Brody JR, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol 2020;21:508-18. [Crossref] [PubMed]
Cite this article as: Gower A, Gresham G, Spector K, Haladjian N, Lee J, Mehta S, Osipov A, Hendifar A. Association of germline ATM mutations and survival in pancreatic cancer. Ann Pancreat Cancer 2021;4:1.