Solid pseudopapillary neoplasm of the pancreas: cytomorphology, pitfalls, and literature review
Introduction
SPN of the pancreas is considered a rare and uncommon neoplasm. First described in the literature by Frantz and then so-called Gruber-Frantz tumor (1). It is mostly seen in young females with a mean age of 25 to 30 years (2). High prevalence of SPN of the pancreas in women suggests a hormonal role in pathogenesis of this tumor (3). Cases occurred in men is very rare (<7% of cases) (4). It usually has no specific anatomical predilection. Unusual presentation like multicentricity or extra-pancreatic presentations were also described in the literature (5-8). The frequency of this tumor varies and ranges up to 2.7% of exocrine pancreatic neoplasms (9). Literature review showed very few of these cases were diagnosed on cytologic specimens (10). Symptoms could range from abdominal discomfort, occasional mass or even incidental finding during imaging study. Uncommonly, local invasion and infiltration of the surrounding capsule may occur. Distant metastasis is very rare (11). As a general rule, SPN is considered a tumor with uncertain malignant potential. The treatment of choice is complete surgical excision and even in those with capsular invasion, is highly responsive to cure (12). There are few reports that some tumors may metastasize (13). The most common metastatic locations include adjacent structures or pleura. When metastasis is discovered, term “carcinoma” is used instead of “neoplasm”. Even those that metastasize have excellent prognosis (14).
Clinical presentation
Generally, abdominal pain, early satiety, nausea, vomiting or even incidental finding during imaging studies of abdomen for other reasons are the non-specific initial clinical presentations (15).
Typically, SPN patient presents as a young woman with few weeks of history of episodic epigastric and left upper quadrant vague abdominal pain. B symptoms like weight loss and fever are uncommon. Imaging studies of the abdomen mostly reveals a pancreatic mass at the body or tail of the pancreas.
Histogenesis
It appears that the histogenesis of SPN is uncertain. Multiple proposed theories include originating from totipotent stem cells or primitive cells that could differentiate to both exocrine and endocrine cells or even deriving from small duct epithelium or acinar cells (16,17). In addition, presence of pre-melanosomes and melanosome granules suggested derivation from neural crest origin (18). More researches are required to better understand the histogenesis of this rare tumor.
Cytomorphology
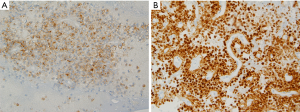
The aspirated material during EUS-FNA usually smeared onto glass slides, air-dried and then immediately stained with hematoxylin for specimen adequacy and preliminary diagnosis. Other smears are fixed in 95% alcohol for PAP staining. The rest of the aspirated material is usually formalin fixed and paraffin embedded to make a cell block for ancillary studies. Fine needle aspiration of a SPN mass-lesion usually provides a highly cellular smear composed of monotonous population of cuboidal cells that are loosely cohesive or single isolated cells. The tumor cells may arrange as single or multiple layers around a fibrovascular core, but this type of appearance is uncommon in cytology smears. Figure 1 shows an endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) biopsy from a patient with pre-operational suspicion of having SPN. The smears are highly cellular. Many single or small clusters of loosely cohesive monotonous tumor cells and few delicate papillary fronds with fibrovascular core lined by two to three layers of cuboidal cells identified. No significant atypia was noted. Tumor cells had round to oval nuclei with fine chromatin. Some tumor cells clearly had nuclear grooves. Tumor cell cytoplasm was mostly clear and poorly defined.
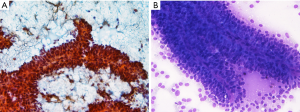
Naked capillaries could also be seen (19). The tumor cells have granular cytoplasm with indistinct cell borders. The nuclei are round-to-oval, having nuclear groove and indistinct nucleoli. The neoplastic cell lining may show some anisocytosis with round-to-oval nuclei and fine chromatin. The background of cytologic smear is usually bloody with amorphous myxoid material and necrotic debris (20). One study also pointed on cercariform cells as another cytologic feature that could be present in SPN cytology and help to distinguish it from pancreatic endocrine neoplasms and acinar cell carcinomas (21). Figure 2 shows the cell block of an EUS-FNA biopsy of a SPN.
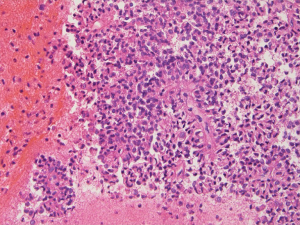
Gross and histologic findings
SPN of the pancreas usually presents as a well-circumscribed mass that could have both solid and non-septated cystic components. Calcification and necrosis can sometimes present. Histologically the tumor is composed of solid and pseudopapillary components that are mixed together. A fibrovascular core is a prominent feature of the pseudopapillary component. The tumor cells are poorly cohesive, monomorphic cells with eosinophilic cytoplasm and nuclear groves. Rare mitosis may be seen. Background stroma could have foam cells with cholesterol clefts (22).
Immunohistochemistry
SPN is immunoreactive for α1-antitrypsin, α1-antichymotrypsin, PR, CD56, CD10 and CD117 (23,24). Cytokeratin can be positive but the stain is weak and focal. Nuclear staining for β-catenin is also a very useful marker. Sometimes the tumor cells may be focally immunoreactive with synaptophysin. Figure 3 shows an immunohistochemical example of a SPN, in which the tumor cells are diffusely positive with CD10 and strong, diffuse nuclear staining with β-catenin.
Molecular findings
Many studies have performed to detect molecular changes in SPN. It has been proven that variations in SMAD4, KRAS, TP53 and CDKN2A, which are common molecular changes in other pancreatic cancers, are not detected in SPN (25). On the other hand, it is confirmed that all the patients with SPN have activating somatic mutation of β-catenin gene (CTNNB1) which is located on chromosome 3p (26). Up to 70% of CTNNB1 mutations are detected on position 32 or 37 (27). In addition, it has been found that some proteins involving in Wnt signaling including DKK4 and some other proteins that function directly through binding with β-catenin protein like; NONO and FUS, are upregulated in SPN (28). Some studies also pointed out that there is a higher presence of single nucleotide polymorphism (SNPs) seen in those SPN patients who are older or having a larger tumor (>100 mm in diameter) or a metastasis (29). Inactivated mutations of epigenetic regulators (e.g., KDM6A, TET1, BAP1) with loss or reduction of their related proteins are also associated with SPN with metastatic lesions and appears to occur before metastasis happens (30). The importance of understanding these mutations is that they may be used as a treatment option in the future by increasing the BAP1 and KDM6A function to decrease the risk of metastasis.
Differential diagnosis
The main differential diagnosis includes acinar cell carcinoma and pancreatic endocrine neoplasms. Papillary mucinous carcinoma and intraductal papillary mucinous tumor could rarely come into the differential diagnosis list. These tumors may share some cytological features that may overlap with SPN. On cytology specimen, pancreatic endocrine neoplasms have single or small clusters of monomorphic plasmacytoid cells with typical salt and pepper chromatin and may make rosettes but no papillary structures (31). On the other hand, acinar cell carcinoma cytology specimen is usually highly cellular with numerous isolated cells. The tumor cells have round-to-oval nucleus with smooth nuclear contours and prominent nucleolus. Tumor cells may have a granular cytoplasm.
Presence of thick mucus helps to differentiate intraductal papillary mucinous tumor from SPN. Also, columnar mucinous cells with architectural atypia are present. In papillary mucinous carcinoma tumor cells have cytoplasmic vacuoles with mucinous background (32,33).
Current guidelines on the management of pancreatic cysts
The guideline for the management of pancreatic neoplastic cysts including SPN has recently been updated. A conservative approach is usually the management of choice for asymptomatic mucinous cystic neoplasm (MCN) and intraductal papillary mucous neoplasm (IPMN) that measure less than 40 mm with no enhancing nodule (34). IPMN with adjacent main pancreatic duct measured between 5 to 9.9 mm or the cyst measures more than 40 mm, is a relative indication of surgery. Because of high risk of malignancy, presence of an enhanced mural nodule of more than 5 mm or main pancreatic duct diameter of more than 10 mm, are absolute indications of surgery (34). Radical resection is also considered the management of choice for all SPN. And in cases of SPN with metastasis, recurrence or locally advanced cases, an aggressive surgical approach is warranted and makes a better long-term outcome (35,36). Neoadjuvant therapy is routinely recommended for IPMN or MCN-associated carcinoma but not for SPN but may be used as a palliative measurement in post-surgical recurrence patients (37). Some studies recommended that if the SPN is unrespectable, it can be preoperatively treated with chemotherapy like with gemcitabine to decrease the size of the lesion before doing the surgery (38). In addition, SPN is a radiosensitive tumor and radiotherapy can be considered as a treatment option in unrespectable tumors (39).
American Society of Clinical Oncology recommends gene testing roles for those individuals who are susceptible for pancreatic cancers (e.g., with blood relative diagnosed with pancreatic cancer) (40). Even in individuals without a defined genetic mutation but having family risk criteria, surveillance is recommended and the consensus is around age 50 (41). The surveillance protocol could include imaging like EUS as the primary modality of choice (42).
Conclusions
It is very important to distinguish SPN from other pancreatic mass tumors most specifically from its main differential diagnosis like acinar cell carcinoma and islet cell tumors. Appropriate surgical management and better prognosis differentiates this tumor from other pancreatic lesions. SPN can be diagnosed preoperatively with fine needle aspiration biopsy (43). This shows the significant role of cytology evaluation for definitive diagnosis and further management. Cytomorphology criteria including hypercellular smear, papillary fronds, fibrovascular core, monomorphic appearance of the lining tumor cells, nuclear grooves, occasional cercariform cells, and background foamy macrophages with necrotic debris are important diagnostic clue. Mitotic figures are usually uncommon in tumor cells. Amorphous myxoid material that appears red following Giemsa staining could be another hint for diagnosis (44). By immunohistochemistry the tumor cells are reactive to α1-antitrypsin, CD56 and CD10. Nuclear staining with β-catenin is very helpful to differentiate it from pancreatic neuroendocrine neoplasm. Progesterone receptors staining has also been described in the literature that could suggest a hormone-dependent role in SPN (44). Fine needle aspiration cytology is the most helpful and cost-effective approach for diagnosis and to plan for surgical management. The diagnostic accuracy of EUS-FNA is superior and more sensitive compared to imaging studies for making the diagnosis (94% compared to 69–83% respectively), especially for lesions less than 3 cm (45). A pre-operative accuracy is highly possible by EUS-guided FNA cytology. EUS-guided FNA can differentiate SPN from certain pancreatic neoplasms with similar imaging findings but with different clinical behavior, treatment and prognosis including acinar cell carcinoma and pancreatic neuroendocrine neoplasm.
In conclusion, EUS-FNA by providing high cellular material and high sensitivity is the modality of choice for pre-operative diagnosis of SPN. We strongly believe and suggest to use this approach by reviewing cytomorphologic features in conjunction with clinic-radiologic findings to make the accurate diagnosis.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-20-42). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pettinato G, Manivel JC, Ravetto C, et al. Papillary cystic tumor of the pancreas. A clinicopathologic study of 20 cases with cytologic, immunohistochemical, ultrastructural, and flow cytometric observations, and a review of the literature. Am J Clin Pathol. 1992;98:478-88. [Crossref] [PubMed]
- Kosmahl M, Pauser U, Peters K, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 2004;445:168-78. [Crossref] [PubMed]
- Wrba F, Chott A, Ludvik B, et al. Solid and cystic tumor of the pancreas; a hormonal-dependent neoplasm? Histopathology 1988;12:338-40. [PubMed]
- Pettinato G, Di Vizio D, Manivel JC, et al. Solid-pseudopapillary tumor of the pancreas: a neoplasm with distinct and highly characteristic cytological features. Diagn Cytopathol 2002;27:325-34. [Crossref] [PubMed]
- Young NA, Villani MA, Khoury P, et al. Differential diagnosis of cystic neoplasms of the pancreas by fine-needle aspiration. Arch Pathol Lab Med 1991;115:571-7. [PubMed]
- Orlando CA, Bowman RL, Loose JH. Multicentric papillary-cystic neoplasm of the pancreas. Arch Pathol Lab Med 1991;115:958-60. [PubMed]
- Tornóczky T, Kálmán E, Jáksó P, et al. Solid and papillary epithelial neoplasm arising in heterotopic pancreatic tissue of the mesocolon. J Clin Pathol 2001;54:241-5. [Crossref] [PubMed]
- Klöppel G, Maurer R, Hofmann E, et al. Solid-cystic (papillary-cystic) tumours within and outside the pancreas in men: report of two patients. Virchows Arch A Pathol Anat Histopathol 1991;418:179-83. [Crossref] [PubMed]
- Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg 2005;200:965-72. [Crossref] [PubMed]
- Pailoor K, Kini H, Rau AR, et al. Cytological diagnosis of a rare case of solid pseudopapillary neoplasm of the pancreas. J Cytol 2010;27:32-4. [Crossref] [PubMed]
- Bondeson L, Bondeson AG, Genell S, et al. Aspiration cytology of a rare solid and papillary epithelial neoplasm of the pancreas. Light and electron microscopic study of a case. Acta Cytol 1984;28:605-9. [PubMed]
- Shuja A, Alkimawi KA. Solid pseudopapillary tumor: a rare neoplasm of the pancreas. Gastroenterol Rep (Oxf) 2014;2:145-9. [Crossref] [PubMed]
- Cappellari JO, Geisinger KR, Albertson DA, et al. Malignant papillary cystic tumor of the pancreas. Cancer 1990;66:193-8. [Crossref] [PubMed]
- Buetow PC, Buck JL, Pantongrag-Brown L, et al. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology 1996;199:707-11. [Crossref] [PubMed]
- Wright MJ, Javed AA, Saunders T, et al. Surgical resection of 78 pancreatic solid pseudopapillary tumors: a 30-year single institutional experience. J Gastrointest Surg 2020;24:874-81. [Crossref] [PubMed]
- Stömmer P, Kraus J, Stolte M, et al. Solid and cystic pancreatic tumors. Clinical, histochemical, and electron microscopic features in ten cases. Cancer 1991;67:1635-41. [Crossref] [PubMed]
- Yagihashi S, Sato I, Kaimori M, et al. Papillary and cystic tumor of the pancreas. Two cases indistinguishable from islet cell tumor. Cancer 1988;61:1241-7. [Crossref] [PubMed]
- Chen C, Jing W, Gulati P, et al. Melanocytic differentiation in a solid pseudopapillary tumor of the pancreas. J Gastroenterol 2004;39:579-83. [Crossref] [PubMed]
- Salla C, Chatzipantelis P, Konstantinou P, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid pseudopapillary tumor of the pancreas: a case report and literature review. World J Gastroenterol 2007;13:5158-63. [Crossref] [PubMed]
- DeLellis RA, Loyd RV, Heitz PU, et al. 3rd ed. World Health Organization classification of tumors, pathology and genetics of tumors of endocrine organs. Lyon, France: IARC press, 2004.
- Samad A, Shah AA, Stelow EB, et al. Cercariform cells: another cytologic feature distinguishing solid pseudopapillary neoplasms from pancreatic endocrine neoplasms and acinar cell carcinomas in endoscopic ultrasound-guided fine-needle aspirates. Cancer Cytopathol 2013;121:298-310. [Crossref] [PubMed]
- Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: A typically cystic carcinoma of low malignant potential. Semin Diagn Pathol. 2000;17:66-80. [PubMed]
- Pettinato G, Manivel JC, Ravetto C, et al. Papillary cystic tumor of the pancreas. A clinicopathologic study of 20 cases with cytologic, immunohistochemical, ultrastructural, and flow cytometric observations, and a review of the literature. Am J Clin Pathol 1992;98:478-88. [Crossref] [PubMed]
- Notohara K, Hamazaki S, Tsukayama C, et al. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol 2000;24:1361-71. [Crossref] [PubMed]
- Cavard C, Audebourg A, Letourneur F, et al. Gene expression profiling provides insights into the pathways involved in solid pseudopapillary neoplasm of the pancreas. J Pathol 2009;218:201-9. [Crossref] [PubMed]
- Park M, Kim M, Hwang D, et al. Characterization of gene expression and activated signaling pathways in solid-pseudopapillary neoplasm of pancreas. Mod Pathol 2014;27:580-93. [Crossref] [PubMed]
- Rodriguez-Matta E, Hemmerich A, Starr J, et al. Molecular genetic changes in solid pseudopapillary neoplasms (SPN) of the pancreas. Acta Oncol 2020;59:1024-7. [Crossref] [PubMed]
- Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut 2009;58:1410-8. [Crossref] [PubMed]
- Guo M, Luo G, Jin K, et al. Somatic Genetic Variation in Solid Pseudopapillary Tumor of the Pancreas by Whole Exome Sequencing. Int. J. Mol. Sci 2017;18:81. [Crossref] [PubMed]
- Amato E, Mafficini A, Hirabayashi K, et al. Molecular alterations associated with metastases of solid pseudopapillary neoplasms of the pancreas. J Pathol 2019;247:123-134. [Crossref] [PubMed]
- Collins BT, Saeed ZA. Fine needle aspiration biopsy of pancreatic endocrine neoplasms by endoscopic ultrasonographic guidance. Acta Cytol 2001;45:905-7. [PubMed]
- Stelow EB, Stanley MW, Bardales RH, et al. Intraductal papillary-mucinous neoplasm of the pancreas. The findings and limitations of cytologic samples obtained by endoscopic ultrasound-guided fine-needle aspiration. Am J Clin Pathol 2003;120:398-404. [Crossref] [PubMed]
- Naresh KN, Borges AM, Chinoy RF, et al. Solid and papillary epithelial neoplasm of the pancreas. Diagnosis by fine needle aspiration cytology in four cases. Acta Cytol 1995;39:489-93. [PubMed]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018;67:789-804. [Crossref] [PubMed]
- Kim MJ, Choi DW, Choi SH, et al. Surgical treatment of solid pseudopapillary neoplasms of the pancreas and risk factors for malignancy. Br J Surg 2014;101:1266-71. [Crossref] [PubMed]
- Wang WB, Zhang TP, Sun MQ, et al. Solid pseudopapillary tumor of the pancreas with liver metastasis: Clinical features and management. Eur J Surg Oncol 2014;40:1572-7. [Crossref] [PubMed]
- Hofmann H, Von Haken R, Werner J, et al. Unresectable isolated hepatic metastases from solid pseudopapillary neoplasm of the pancreas: a case report of chemosaturation with high-dose melphalan. Pancreatology 2014;14:546-9. [Crossref] [PubMed]
- Maffuz A, Bustamante Fde T, Silva JA, et al. Preoperative gemcitabine for unresectable, solid pseudopapillary tumour of the pancreas. Lancet Oncol 2005;6:185-6. [Crossref] [PubMed]
- Fried P, Cooper J, Balthazar E, et al. A role for radiotherapy in the treatment of solid and papillary neoplasms of the pancreas. Cancer 1985;56:2783-5. [Crossref] [PubMed]
- Park M, Lim JS, Lee HJ, et al. Distinct Protein Expression Profiles of Solid-Pseudopapillary Neoplasms of the Pancreas. J Proteome Res 2015;14:3007-14. [Crossref] [PubMed]
- Goggins M, Overbeek KA, Brand RInternational Cancer of the Pancreas Screening (CAPS) consortium, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69:7-17. [Crossref] [PubMed]
- Wani S, Han S, Simon V, et al. Setting minimum standards for training in EUS and ERCP: results from a prospective multicenter study evaluating learning curves and competence among advanced endoscopy trainees. Gastrointest Endosc 2019;89:1160-8.e9. [Crossref] [PubMed]
- Katz LB, Ehya H. Aspiration cytology of papillary cystic neoplasm of the pancreas. Am J Clin Pathol 1990;94:328-33. [Crossref] [PubMed]
- Rosai J. Pancreas and periampullary region. In: Rosai J. editor. Ackerman’s surgical pathology. 9th ed. St. Louis, Missouri: Mosby, 2004:1061-14.
- Müller MF, Meyenberger C, Bertschinger P, et al. Pancreatic tumors: evaluation with endoscopic US, CT, and MR imaging. Radiology 1994;190:745-51. [Crossref] [PubMed]
Cite this article as: Pahlavan PS, Khiyami A, Ganesan S. Solid pseudopapillary neoplasm of the pancreas: cytomorphology, pitfalls, and literature review. Ann Pancreat Cancer 2021;4:5.