A Phase 1b clinical trial of LDE225 (Sonidegib) in combination with fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFIRINOX) in previously untreated locally advanced or metastatic pancreatic adenocarcinoma
Introduction
Pancreatic cancer is the 3rd leading cause of cancer related deaths in the USA (1). The only known curative approach remains surgical resection (2). However, the majority of patients present with locally advanced or metastatic disease and are not candidates for surgery. For patients with advanced disease, the median overall survival (OS) remains less than 1 year (3). Although survival of patients with advanced disease has improved in the past decade, the prognosis for most patients remains poor due to modest efficacy of systemic therapy. Development of novel treatment approaches with enhanced effectiveness are critical for improving the outcome of these patients.
Over the past decade, two regimens have been developed which have improved the OS of patients with metastatic pancreatic cancer. The first to be established was a combination of fluorouracil (5-FU), leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) (4). A randomized phase III trial compared FOLFIRINOX to gemcitabine in patients with metastatic pancreatic adenocarcinoma. Doses of chemotherapy were: oxaliplatin 85 mg/m2; leucovorin 400 mg/m2; irinotecan 180 mg/m2; 5-FU 400 mg/m2 bolus; and 5-FU 2,400 mg/m2 by 46-h infusion. The regimen was administered every 14 days. Toxicities of FOLFIRINOX, which were manageable, included a 12.7% rate of grade 3/4 diarrhea, 45.7% grade 3/4 neutropenia, 5.4% febrile neutropenia, and 9.1% thrombocytopenia. FOLFIRINOX used in the first-line metastatic setting produced response rates of 31.6% as compared to 9.4% (P<0.001) for gemcitabine. Median progression-free survival (PFS) was 6.4 months for FOLFIRINOX compared to 3.3 months for gemcitabine. The median OS was 11.1 months for FOLFIRINOX versus 6.8 months for gemcitabine (P<0.001). This established FOLFIRINOX as a new standard in the treatment of pancreatic adenocarcinoma in patients with advanced disease and good performance status. The other regimen that also improved survival compared to gemcitabine alone was the combination of gemcitabine and nab-paclitaxel (5). However, given that the median OS for patients with metastatic disease remains less than 1 year, novel treatment approaches are needed.
Several potentially promising targets for improving the treatment of pancreatic cancer have been identified (6-8). Among these are the Hedgehog signaling pathway. Secreted Sonic Hedgehog (SHH) is a ligand with wide-ranging concentration-dependent effects on embryonic development, in addition to its role in cancer (8,9). The underlying mechanism of hedgehog signaling involves Patched 1 (PTCH1), which inhibits the G-protein coupled receptor Smoothened (Smo) by preventing its localization to the cell surface. Upon binding of SHH to its receptor PTCH1, the PTCH1 complex is internalized and releases repression of Smo. Smo localizes to the surface and initiates signaling that leads to activation of the glioma-associated (Gli) family of zinc-finger transcription factors. This in turn leads to upregulation of hedgehog-specific genes, including PTCH1 and Smo.
The hedgehog pathway can be activated in cancer in two ways: mutation in pathway components such as PTCH1 (such as can occur in medulloblastoma), or overexpression of SHH ligand (10). For example, overexpression of the hedgehog ligand has been found in a significant percentage of human pancreatic cancers and in pancreatic cancer precursor lesions. In addition, pancreatic-specific expression of hedgehog in transgenic mice led to histologic changes consistent with the development of pancreatic cancer; and, pancreatic cancer cell lines expressing components of the hedgehog pathway were sensitive to cyclopamine, a steroidal alkaloid that inhibits Smo (10). Rather than a direct role of the hedgehog pathway on pancreatic cancer cell proliferation, other studies have demonstrated the ligand-dependent role of the hedgehog pathway in epithelial tumors, and demonstrated the importance of a paracrine effect of SHH on the tumor microenvirinoment including stromal and endothelial components of the tumor (11,12). This mechanism is thought to be particularly relevant to pancreatic cancer, given that these tumors possess a large stromal component. Inhibiting this activity with neutralizing monoclonal antibody had antitumor effect preclinically (13). Based on the hypothesis that the stromal milieu may impede delivery of chemotherapy to pancreatic tumors, thus possibly explaining the limited response of pancreatic cancers to chemotherapy, hedgehog inhibition with a Smo antagonist improved delivery of gemcitabine chemotherapy to tumors, as compared to mice treated with gemcitabine alone (14). Furthermore, pancreatic tumors from Smo-inhibitor-treated mice had a marked decrease in desmoplastic stroma and showed an increase in microvessel density compared to tumors from mice treated with gemcitabine alone. Finally, genetically engineered mice with pancreatic cancers lived longer after treatment with gemcitabine plus a Smo-inhibitor as compared to control gemcitabine-treated mice supporting a role for hedgehog pathway inhibition in the improved delivery of chemotherapy for pancreatic cancer. The high stromal nature of pancreatic adenocarcinomas, the upregulation of SHH signals in human pancreatic cancers, evidence indicating a paracrine role of SHH in stromal pathway signals, and the availability of SHH inhibitors that showed anticancer activity in pancreatic cancer models all pointed to further clinical study of hedgehog pathway inhibition in pancreatic adenocarcinoma.
The first Smo inhibitor to be approved for use against cancer was Vismodegib which has been approved for use in the treatment of locally advanced or metastatic basal cell carcinoma (15). LDE225 (Sonidegib) is a potent selective and orally bioavailable SMO antagonist from the structural class N-[6-(cis-2,6-dimethylmorpholin-4-yl)pyridine-3-yl]-2-methyl-4’-(trifluoromethoxy)-1,1’-[biphenyl]-3-carboxamide diphosphate that has been shown to inhibit SHH-and Smo-dependent proliferation in vivo. LDE225 has been investigated in a number of clinical trials in patients with cancer and has subsequently been approved for use in the treatment of patients with locally advanced or metastatic basal cell carcinoma (16). This study evaluated whether targeting the stroma of pancreatic tumors with LDE225 was feasible and would enhance the response rate to FOLFIRINOX chemotherapy.
Methods
Study design
This multicenter, single-arm phase 1b trial enrolled patients with histologically confirmed locally advanced or metastatic pancreatic cancer who had not received prior systemic chemotherapy for locally advanced or metastatic disease. Prior adjuvant chemotherapy and/or radiation therapy were allowed. The trial was a dose escalation study and was conducted at Massachusetts General Hospital (MGH) and Dana Farber Cancer Institute (DFCI) through the Dana Farber/Harvard Cancer Center consortium. Patients received FOLFIRINOX [starting doses of the chemotherapeutic agents in FOLFIRINOX depended on the dose level which the patient was on (Table S1)] on days 1–3 of a 14-day cycle. LDE225 was given orally every day. The dose of LDE225 depended on the dose level the patient received (Table S1). The primary objectives were to determine the: maximum tolerated doses (MTDs) of LDE225 in combination with FOLFIRINOX in patients with locally advanced or metastatic pancreatic adenocarcinoma; and, the toxicity and safety profile of LDE225 in combination with FOLFIRINOX. Other objectives included determining the response rate of pancreatic cancers treated with LDE225 in combination with FOLFIRINOX and characterizing OS, PFS, and whether any patients with locally advanced disease were converted to resectable disease.
Investigators at the MGH Cancer Center designed the study. Study data was collected and analysed by the study investigators. The study protocol and all related study documents were approved by the DFCI Institutional Review Board. The study was conducted in accordance with the International Conference on Harmonization and Good Clinical Practice (ICH-GCP) and the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Dana Farber Cancer Institute (DFCI-IRB 44-417). All patients were required to give written informed consent before enrolment. The trial was registered in clinicaltrials.gov, identifier: NCT01485744
Study patient selection
Subjects had to have measurable disease, per RECIST 1.1 criteria; performance status 0–1; age ≥18 years; life expectancy of greater than 12 weeks; have adequate organ and marrow function as defined by: absolute neutrophil count ≥1,500/mcL; platelets ≥125,000/mcL; total bilirubin ≤1.5× normal institutional limits; aspartate aminotransferase (AST) (SGOT) and alanine aminotransferase (ALT) (SGPT) ≤3× normal institutional limits, or ≤5× if liver metastases are present; creatinine ≤2× normal institutional limits; plasma creatinine phosphokinase <1.5 normal institutional limits; QTc interval ≤470 ms on screening ECG. Patients with CNS disease were allowed to participate provided that whole brain radiotherapy had been received not less than 4 weeks prior to starting the study drug and the stability of the brain metastasis had been demonstrated. Patients who were hypersensitive to any component of the treatment regimen were excluded. Patients could not have a concurrent active primary or metastatic cancer other than superficial squamous cell or basal cell skin cancer. HIV-positive individuals on combination antiretroviral therapy were ineligible because of the potential for pharmacokinetic interactions with the treatment regimen, and because of the risk for leukopenia/neutropenia.
Study treatment
The initial LDE225 dose at dose level 1 was 200 mg PO daily. The doses of chemotherapy at dose level 1 were as follows: oxaliplatin 85 mg/m2 over 2 hours; leucovorin 400 mg/m2 over 2 hours; irinotecan 180 mg/m2 in 90 min infusion; 5-FU 400 mg/m2 bolus; 5-FU 2,400 mg/m2 by 46-h infusion. The regimen was administered every 14 days. Several levels were designed for both dose escalation, and because of the potential for toxicities that could prevent continuing treatment at the initial dose level, several dose de-escalation levels were also included (see Table S1).
Safety monitoring
During the course of study, all patients were closely evaluated for toxicity and disease assessment. Patients were monitored and assessed for toxicity prior to and during every cycle for adverse events (AEs) according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver 4.0.
A history was taken, vital signs, physical examination and laboratory evaluations (complete blood counts with differentials and complete metabolic profile) were performed before each dose of FOLFIRINOX chemotherapy. Patients were evaluated for their compliance to study drug use as per protocol. They were also monitored for any dose reduction or modification. The trial was independently monitored and assessed by the DFCI/Harvard Cancer Center Data and Safety Monitoring Committee (DSMC).
Tumour response evaluation
Tumor response was performed using contrast enhanced computed tomography (CT) scans and evaluated as per the RECIST ver1.1. CT scans were performed at baseline and then after every four cycles. Trial participants who received at least one cycle of therapy were eligible for evaluation. The independent DFCI/Harvard Cancer Center radiological review [tumor imaging metrics core (TIMC)] did the response evaluations.
Patient follow up
All study participants were followed for survival from the last dose of study drug every 3 months until the subject’s death or loss to follow up. Vital status was verified at clinic visits or telephone contacts.
Statistical methods and analysis
The primary objectives were to determine MTDs of LDE225 in combination with FOLFIRINOX in patients with locally advanced or metastatic pancreatic adenocarcinoma and to assess the toxicity and safety profile of the combined treatment. A standard dose-escalation schema was utilized with cohorts of three subjects per dose level. The dose-limiting toxicity (DLT) monitoring period was three cycles (or 42 days) for the initial three patients treated at a given dose level and two cycles (or 28 days) for the subsequent three patients at a given dose level. The occurrence of one (DLT) prompted expansion of a given dose level to six subjects. The occurrence of two DLTs indicated that the MTD had been exceeded, resulting in expansion of the prior dose level to six subjects if not already performed. The MTD was defined as the highest dose level at which less than 33% of the patients experience a DLT. All participants who received one dose were evaluable for toxicity from the time of their first treatment.
Secondary objectives included estimating the response rate, the number/rate of locally advanced patients converted to resectable disease, and characterizing OS and PFS. Once the MTD was established, an additional 16 patients (total of 22 patients at the MTD) were enrolled into the expanded cohort in order to further explore the efficacy and safety of the combination when dosed at the MTD. PFS was defined as the time from enrollment to disease progression or death from any cause, and OS was defined as the time from enrollment to death from any cause. PFS and OS times for patients without the event of interest were censored at the date of last follow-up. Distributions of PFS and OS were summarized using the Kaplan-Meier method.
Results
Patient characteristics
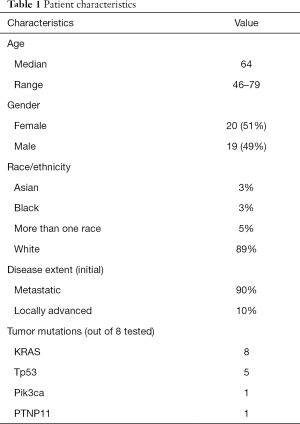
Among 39 patients enrolled, 35 received at least 1 dose of combined FOLFIRINOX and LDE225. Characteristics of the patients included: 51% female; median age 64 years; 3% Black, 3% Asian, 5% more than one race, and 89% White; 90% of the patients had metastatic disease at presentation, while 10% had locally advanced disease, although all of these ultimately developed metastatic disease; 97% had not received prior therapy for locally advanced or metastatic disease. Molecular characterization of the tumor was not required by the study. Eight patients had analysis done using the version of the MGH tumor genetic analysis snapshot assay current at the time they enrolled (17). All eight tumors had KRAS mutations, there were five p53 mutations, one had a PIK3CA mutation, and one had a PTNP11 mutation (Table 1).
Full table
Determination of MTD
From the initial doses (level 1), de-escalation occurred three times before the MTD was established at dose level-2A. The MTD doses were:
- Daily: LDE225, 400 mg PO daily;
- Day 1: irinotecan 120 mg/m2;
- Day 1: oxaliplatin 65 mg/m2;
- Day 1: omit 5-FU bolus; administer leucovorin 400 mg/m2 bolus;
- Day 1–2: 5-FU 1,800 mg/m2 46-h continuous infusion.
Toxicity assessment
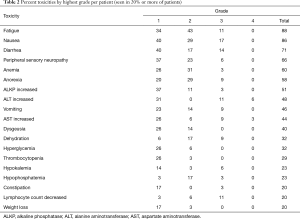
For patients who received at least one cycle of therapy, drug-related AEs occurring in at least 50% of the patients were: fatigue (88%), nausea (86%), diarrhea (71%), peripheral sensory neuropathy (66%), anemia (60%), anorexia (57%), and elevated alkaline phosphatase (ALKP) (51%). Elevated ALT occurred in 49% of patients and elevated AST in 43%. The most common grade 3 toxicities were nausea (17%), diarrhea (14%), fatigue (11%), dehydration (9%), AST elevation (9%), and ALT elevation (9%). Four patients (11%) had grade 4 AEs (two elevated ALT, one elevated AST, one hypoglycemia). There were no grade 5 events. Table 2 lists all toxicities that occurred in at least 20% of the patients.
Full table
Tumour response and survival
At the MTD, a total of 18/22 (85%) patients completed at least one cycle of treatment and were evaluable for response. Six of 18 patients (33%) stayed on therapy for at least 6 months and 2 patients (11%) were on therapy for over 1 year. One patient was on therapy for 39 months before progressing.
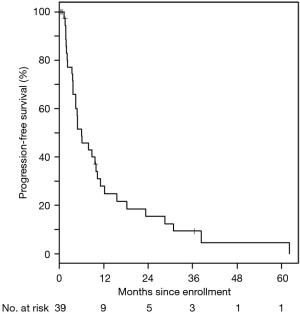
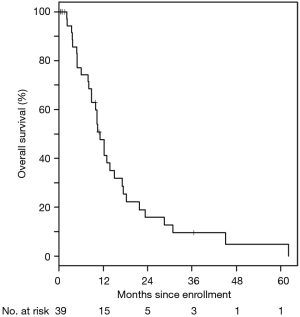
For the entire cohort, 35/39 (90%) of patients received at least one cycle of treatment and 26 were evaluable for response. The best response was partial in 31% of patients, stable disease in 46%, and progressive disease in 23%. Ten (29%) stayed on treatment for at least 6 months, four (11%) were on therapy over 1 year, and three (9%) stayed on therapy over 2 years, with the longest patient on therapy for 39 months. The median PFS was 6.1 (95% CI: 3.8–9.9) months (Figure 1). The median OS was 11.2 (95% CI: 8.8–15.0) months (Figure 2). These are both similar to those reported for FOLFIRINOX alone (4).
Best responder
The patient who had the best response was a 78-year-old woman who initially had the following presentation. She reported that food tasted poorly, she was experiencing early satiety, nausea and vomiting, loose stools, dry mouth with excessive thirst and then abdominal and back pain. She had a 37-pound weight loss over 6 months. Abdominal MRI revealed over fifteen enhancing lesions throughout the liver which demonstrate restricted diffusion, multiple enlarged portahepatic lymph nodes, and a 3.6-cm pancreatic head mass. Cytology from a FNA of the pancreatic head mass revealed well differentiated adenocarcinoma. Molecular testing revealed a KRAS mutation in codon 12, two TP53 mutations, and a mutation in PTNP11. The tumor was microsatellite stable. CA19-9 was elevated at 40. She had a good initial response including prompt normalization of her CA19-9 and then a continued response over time. At the time of maximum response, the only detectable disease was an ill-defined soft tissue density measuring approximately 1.1 cm in the head of the pancreas and she therefore remained a partial response. Unfortunately, she developed recurrent metastatic disease at 39 months and came off of the study at that point.
Conclusions
Although improvements have been made in the treatment of patients with metastatic pancreatic cancer over the past decade, especially with the development of two chemotherapy regimens (FOLFIRINOX and gemcitabine plus nab-paclitaxel), median OS with each of these remains less than 1 year so there is a pressing need for improved treatment approaches. Significant preclinical evidence suggests that the tumor microenvironment plays a significant role in the development and ongoing maintenance of pancreatic cancer. Although the pancreatic tumor microenvironment is complex with interactions between malignant, inflammatory, immune, and vascular cells as well as secreted proteins and extracellular matrix components, one of the findings that is characteristic of pancreatic cancers is the significant degree of desmoplasia and fibrosis seen (18). Preclinical studies have suggested that this inhibits delivery of adequate chemotherapy to cancer cells. A number of different factors and pathways are involved in development of desmoplasia and fibrosis. One of these is SHH, which promotes desmoplasia and fibrosis in pancreatic cancers. Preclinical experiments have suggested that inhibition of the SHH pathway could improve chemotherapy delivery to the cancer cells with potential for improved response (14,19).
Based on significant preclinical data supporting potential improved chemotherapy efficacy with inhibition of the SHH pathway, we performed this phase Ib study of combined LDE225 (Sonidegib) with FOLFIRINOX for patients with metastatic or locally advanced pancreatic cancer. Unfortunately, overlapping toxicities of LDE225 with some of those seen with FOLFIRINOX (including fatigue, nausea, vomiting, anorexia, diarrhea, and transaminitis) meant that the doses of each of the chemotherapeutic agents in standard FOLFIRINOX had to be significantly reduced which prevented the ability to test the efficacy of the combination at optimal doses. Overall, the median PFS and OS results of this trial were similar to those seen for FOLFIRINOX alone (Figures 1,2).
Despite significant preclinical research supporting a potential role for SHH inhibition in the treatment of pancreatic cancer, especially in combination with chemotherapy or anti-epidermal growth factor receptor (EGFR) treatment, a number of clinical trials with a variety of different SHH inhibitors combined with either chemotherapy or anti-EGFR inhibition, have failed to show any clear benefit for either SHH inhibition alone or in combination with other agents (20-25). The reasons for this discrepancy between extensive preclinical studies and what has been seen in the clinic are not entirely clear. However, the interactions between pancreatic cancer cells and the tumor microenvironment are complex so that modulation of one pathway may not have the clinical impact in humans that it has in simpler model systems (26). In addition, at least one preclinical study suggested chemoresistance (to gemcitabine and 5-FU) when inhibition of SHH is used in the setting of hypoxic tumors, possibly due to a decrease of cancer cells entering S-phase (27). Importantly, the lack of clinical benefit with the addition of Hedgehog inhibitors to chemotherapy, despite extensive preclinical findings showing benefit for SHH inhibition with chemotherapy for pancreatic cancer, indicates the difficulty in translating preclinical findings to clinical benefit. Similarly, another attempt to target the microenvironment to improve chemotherapy by utilizing the combination of pegylated recombinant human hyaluronidase (pegvorhyaluronidase alfa) with FOLFIRINOX chemotherapy to treat pancreatic cancer also did not produce enhanced efficacy and in fact appeared to be detrimental, again despite preclinical data to support this approach (28). The results of the randomized phase III clinical trial of pegvorhyaluronidase alfa with Gemcitabine and Nab-Paclitaxel also did not show an improvement in OS or PFS, indicating that the absence of benefit was not due to the specific chemotherapy regimen utilized (29). These examples emphasize the difficulty of translating preclinical data to clinical efficacy and the need for both a better understanding of the fundamental biology of the pancreatic cancer cell-microenvironment interaction as well as carefully done preclinical studies based on understanding of the biology of this interaction in humans and not just animal models.
A second important lesson is the difficulty of combining anti-cancer treatment regimens when there are potential overlapping toxicities that require dose reductions of one or both of the components. Part of the success of both FOLFIRINOX and the gemcitabine plus nab-paclitaxel regimens for pancreatic cancer is the ability to give full, or in the case of modified FOLFIRINOX, nearly full doses of each of the agents used individually. In this trial, doses of each of the chemotherapeutic agents (5-FU, irinotecan, and oxaliplatin) in FOLFIRINOX needed to be significantly attenuated in order for concomitant delivery to be tolerated. This has been an issue for combinations of FOLFIRINOX with a number of other agents as well, limiting the ability to fully evaluate the efficacy of these regimens in the clinic. However, combinations of FOLFIRINOX with certain agents, such as monoclonal antibodies against VEGF or EGFR, where overlapping or induced toxicities are not as extensive, allowed full dosing of the agents and have been successful in the treatment of colorectal cancer (30,31). A number of other agents without significant overlapping toxicities have also been successfully used with the FOLFIRINOX regimen or are currently in clinical trials (32). In the future, increased emphasis should be placed on identifying agents with activity against pancreatic cancer that can potentially be combined with FOLFIRINOX or similarly intense chemotherapeutic regimens.
In summary, LDE225 could be combined with FOLFIRINOX with acceptable toxicities, albeit requiring dose reductions of each of the chemotherapeutic agents in FOLFIRINOX. Similar to previous studies with other SHH inhibitors, we did not find any evidence for additional clinical benefit for the combination. Significant new information indicating the potential for benefit of SHH inhibition in treating pancreatic cancer is required before considering future clinical trials testing these inhibitors in the treatment of pancreatic cancer.
Study limitations
There are several limitations to this study. (I) It was a non-randomized single-arm dose finding phase I study with small sample size and an unselected patient population which could have impacted the limited efficacy seen. (II) There is no predictive biomarker to select the patients who may be more likely to be responsive to LDE225 and if there is a responsive subset, this would be diluted by patients whose tumors are unlikely to respond. (III) Overlapping toxicities of LDE225 with some of those seen with FOLFIRINOX meant that the doses of standard FOLFIRINOX had to be significantly reduced which prevented the ability to test the efficacy of the combination at optimal doses. (IV) We were not able to measure whether any changes occurred in the SHH pathway as a result of LDE225 treatment so we cannot be sure that the target was actually modulated in the patient’s tumors.
Acknowledgments
We especially thank the patients and their families as well as the nurses who took care of them making this study possible. We also thank the clinical research associates and especially Eamala Sunduram, whose dedicated efforts were critical for study conduct.
Funding: This work was supported by Novartis through providing the LDE225 and funding for support of the conduct of the clinical trial. JWC was partially funded by P30CA06516 (Benz) and NCI-ASCO Clinical Investigator Team Leadership Supplemental Award (Role: Investigator).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apc-20-41
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apc-20-41). JWC was partially funded by P30CA06516 (Benz) (NCI-ASCO Clinical Investigator Team Leadership Supplemental Award NIH/NCI/DFCI/Subaward to MGH). CSF reports consulting role for Agios, Amylin Pharmaceuticals, Astra-Zeneca, Bain Capital, CytomX Therapeutics, Daiichi-Sankyo, Eli Lilly, Entrinsic Health, Evolveimmune Therapeutics, Genentech, Merck, Taiho, and Unum Therapeutics. He also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health. He is a co-founder of Evolveimmune Therapeutics and has equity in this private company. He has provided expert testimony for Amylin Pharmaceuticals and Eli Lilly outside the submitted work. BMW reports grants and research funding from Eli Lilly and Company, Consultant for Grail, research funding from Celgene, Inc., Consultant for Celgene, Inc., Consultant for BioLineRx, outside the submitted work. JEF reports full-time employee of Novartis from 2015–2019, during the conduct of the study; grants from Curis, grants from Incyte, grants from Tizona, grants from Astellas, grants from H3 Biomedicine, outside the submitted work. JEF was an employee of MGH and Harvard throughout his involvement in the study, then was an employee of Novartis (2015–2019) after leaving Harvard and MGH before he moved to the Dartmouth-Hitchcock Norris Cotton Cancer Center. JAC reports personal fees from Advanced Accelerator Applications, personal fees from Lexicon, personal fees from Ipsen, personal fees from Crinetics, personal fees from Novartis, stock ownership of Merck, outside the submitted work. KN reports grants from National Cancer Institute, grants from Department of Defense, during the conduct of the study; grants and non-financial support from Pharmavite, LLC, non-financial support from Evergrande Group, grants from Janssen, grants from Revolution Medicines, grants from Genentech, grants from Gilead Sciences, personal fees from Seattle Genetics, personal fees from Array Biopharma, personal fees from BiomX, personal fees from X-Biotix Therapeutics, outside the submitted work. TAA reports personal fees from Merck, personal fees from Agios, personal fees from Ipsen, personal fees from Exelixis, personal fees from Bristol Myers Squibb, personal fees from Genentech, Stock Ownership of Biogen Idec, outside the submitted work. DPR reports equity of Acworth Pharmaceuticals, personal fees from MPM Capital, equity of MPM Capital, equity of Thrive Earlier Detection, personal fees from Gritstone Oncology, personal fees from Maverick Therapeutics, personal fees from Johns Hopkins University Press, personal fees from Uptodate, personal fees from McGraw Hill, grants from SU2C, equity of Exact Sciences, outside the submitted work. ELK was an employee of MGH and Harvard throughout her involvement in the study and is currently employed by Novartis, outside the submitted work. TSH reports personal fees from Merck, personal fees from Synthetic Biologics, personal fees from Novocure, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the International Conference on Harmonization and Good Clinical Practice (ICH-GCP) and the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Dana Farber Cancer Institute (DFCI-IRB 44-417). All patients were required to give written informed consent before enrolment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [Crossref] [PubMed]
- Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery Nat Rev Clin Oncol 2019;16:11-26. [Crossref] [PubMed]
- Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet 2020;395:2008-20. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Hiroshima Y, Kasajima R, Kimura Y, et al. Novel targets identified by integrated cancer-stromal interactome analysis of pancreatic adenocarcinoma. Cancer Lett 2020;469:217-27. [Crossref] [PubMed]
- Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol 2020;17:108-23. [Crossref] [PubMed]
- Skoda AM, Simovic D, Karin V, et al. The role of the Hedgehog signaling pathway in cancer: a comprehensive review. Bosn J Basic Med Sci 2018;18:8-20. [Crossref] [PubMed]
- Jeng KS, Chang CF, Lin SS. Sonic Hedgehog signaling in organogenesis, tumors, and tumor microenvironments. Int J Mol Sci 2020;21:758. [Crossref] [PubMed]
- Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 2003;425:851-6. [Crossref] [PubMed]
- Li X, Ma Q, Duan W, et al. Paracrine Sonic Hedgehog signaling derived from tumor epithelial cells: a key regulator in the pancreatic tumor microenvironment. Crit Rev Eukaryot Gene Expr 2012;22:97-108. [Crossref] [PubMed]
- Chen W, Tang T, Eastham-Anderson J, et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci U S A 2011;108:9589-94. [Crossref] [PubMed]
- Michaud NR, Wang Y, McEachern KA, et al. Novel neutralizing hedgehog antibody MEDI-5304 exhibits antitumor activity by inhibiting paracrine hedgehog signaling. Mol Cancer Ther 2014;13:386-98. [Crossref] [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Frampton JE, Basset-Séguin N. Vismodegib: a review in advanced basal cell carcinoma. Drugs 2018;78:1145-56. [Crossref] [PubMed]
- Lear JT, Migden MR, Lewis KD, et al. Long-term efficacy and safety of Sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatol Venereol 2018;32:372-81. [Crossref] [PubMed]
- Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO Mol Med 2010;2:146-58. [Crossref] [PubMed]
- Thomas D, Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer 2019;18:14. [Crossref] [PubMed]
- Mpekris F, Papageorgis P, Polydorou C, et al. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J Control Release 2017;261:105-12. [Crossref] [PubMed]
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a Hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol 2015;33:4284-92. [Crossref] [PubMed]
- Kim EJ, Sahai V, Abel EV, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res 2014;20:5937-45. [Crossref] [PubMed]
- LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res 2011;17:2502-11. [Crossref] [PubMed]
- Ko AH, LoConte N, Tempero MA, et al. A phase I study of FOLFIRINOX plus IPI-926, a Hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 2016;45:370-5. [Crossref] [PubMed]
- De Jesus-Acosta A, Sugar EA, O'Dwyer PJ, et al. Phase 2 study of vismodegib, a hedgehog inhibitor, combined with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic adenocarcinoma. Br J Cancer 2020;122:498-505. [Crossref] [PubMed]
- McCleary-Wheeler AL, Carr RM, et al. Phase 1 trial of Vismodegib and Erlotinib combination in metastatic pancreatic cancer. Pancreatology 2020;20:101-9. [Crossref] [PubMed]
- Crawford HC, Pasca di Magliano M, Banerjee S. Signaling networks that control cellular plasticity in pancreatic tumorigenesis, progression, and metastasis. Gastroenterology 2019;156:2073-84. [Crossref] [PubMed]
- Onishi H, Morifuji Y, Kai M, et al. Hedgehog inhibitor decreases chemosensitivity to 5-fluorouracil and gemcitabine under hypoxic conditions in pancreatic cancer. Cancer Sci 2012;103:1272-9. [Crossref] [PubMed]
- Ramanathan RK, McDonough SL, Philip PA, et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol 2019;37:1062-9. [Crossref] [PubMed]
- Van Cutsem E, Tempero MA, Sigal D, et al. Randomized phase III Trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J Clin Oncol 2020;38:3185-94. [Crossref] [PubMed]
- Chaix M, Vincent J, Lorgis V, et al. FOLFIRINOX bevacizumab is a promising therapy for chemorefractory metastatic colorectal cancer. Oncology 2014;87:148-58. [Crossref] [PubMed]
- Assenat E, Desseigne F, Thezenas S, et al. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist 2011;16:1557-64. [Crossref] [PubMed]
- Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol 2019;5:1020-7. [Crossref] [PubMed]
Cite this article as: Clark JW, Horick N, Allen JN, Blaszkowsky LS, Murphy JE, Fuchs CS, Wolpin BM, Mayer RJ, Farris JE, Chan JA, Ng K, McCleary NJ, Abrams TA, Ryan DP, Kwak EL, Hong TS. A Phase 1b clinical trial of LDE225 (Sonidegib) in combination with fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFIRINOX) in previously untreated locally advanced or metastatic pancreatic adenocarcinoma. Ann Pancreat Cancer 2021;4:2.