A single-institutional analysis of racial disparities in clinicopathologic characteristics, treatment selections, and outcomes in advanced-stage pancreatic cancer patients
Introduction
Approximately 60,000 people will be diagnosed with pancreatic ductal adenocarcinoma (PDA) in the USA in 2021, and it will be the 4th deadliest cancer, independent of sex (1). There are several epidemiological reports that have investigated PDA racial disparities, specifically with regards to risk factors and disease characteristics at presentation. African-American (AA) PDA patients are more often diagnosed at a younger age and a more advanced stage than White patients (2,3). PDA patients of Hawaiian descent may also be more likely to be diagnosed with more aggressive disease compared to White patients (4). There may be sex differences in disparities in risk factors that contribute to the higher risk of PDA in patients of AA descent (5). However, the contribution of known risk factors such as cigarette smoking, diabetes, family history of PDA, and elevated body mass index (BMI) do not appear to entirely account for PDA racial survival disparities (6).
Some studies suggest that disparities in access and utilization of treatment may be a contributing factor to different clinical outcomes in different racial groups. Several reports found that AA patients with early-stage disease are less likely to receive surgery and possibly chemotherapy and/or radiation therapy (7-9). However, the role of chemotherapy in PDA disparities for patients with advanced disease is not entirely understood. One study found that advanced-stage AA patients were less likely to receive chemotherapy than advanced-stage White patients (10), while a more recent report found no racial differences in receipt of chemotherapy for patients diagnosed with advanced disease (7). Several reports suggest that there are no differences in overall survival (OS) between AA and White patients (3,4), while others report inferior OS for AA patients compared to White patients (6,11,12). One report found that racial disparities in survival were dependent upon stage of disease; survival disparities were present for patients diagnosed with early-stage disease but not in those diagnosed with advanced stage disease (7).
There is not a clear consensus in the literature about the prevalence of racial inequities in PDA risk factors, treatment, or survival. Most of these studies previously discussed were conducted prior to a setting of contemporary chemotherapy regimens such as FOLFIRINOX and gemcitabine/nab-paclitaxel, and the patient populations under investigation are from national databases where treatment was received in a variety of institutional settings. These reports are also epidemiological in nature with inherent limitations in the level of depth of analyses, and there are some discrepancies in their findings that warrant further investigation. To test the hypothesis that there might be a difference in clinical outcomes between AA and White PDA patients, we performed an empiric review of racial disparities in clinicopathologic characteristics, treatment, and survival of PDA patients with advanced disease treated at a single quaternary urban medical center in an era of contemporary chemotherapy. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apc-21-5).
Methods
Patients
This project was a retrospective review of all PDA patients treated at a single institution between 2012–2017. The inclusion criteria were documented race (self-reported), age ≥18 years old, histopathologic diagnosis of PDA, advanced, unresectable or metastatic disease, receipt of gemcitabine and/or fluorouracil, and treatment received at a single institution between January 1, 2012 and December 31, 2017. The exclusion criteria were any other cancer diagnosed and curatively treated within 3 years of PDA diagnosis excluding carcinoma in situ, treated basal cell carcinoma, and superficial bladder tumors (Ta, Tis, and T1).
Clinical data
Eligible patients were identified by a centralized institutional database using ICD diagnosis codes. Patient histories were reviewed and data pertaining to PDA diagnosis and treatment was abstracted from electronic medical records. Available demographic and socioeconomic characteristics, status for well-documented PDA risk factors, and clinicopathologic characteristics at baseline were recorded. It is institutional policy that patients designate race on their initial intake forms when they arrive for their first visit with a provider within our health system. Available treatment data including neoadjuvant and adjuvant therapy for primary PDA and treatment for recurrent or metastatic PDA was collected. Treatment data for recurrent or metastatic PDA included first- and second-line chemotherapy drug regimens, duration of therapy, course-altering toxicity, and receipt of palliative radiation. Course-altering toxicity was defined as toxicity that required a significant delay in treatment, an alteration of chemotherapy dose, hospitalization, or cessation of treatment. Last date of follow-up and vital status were also collected for survival analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (NO. 19-01539) and individual consent for this retrospective analysis was waived.
Statistical analysis
To describe the distribution of the covariates, frequency with proportion were calculated for categorical variables and mean with standard deviation or median with range were calculated for continuous variables. Chi-squared or Fisher’s exact tests were used to compare the distribution of categorical covariates in different races and Student’s t-test or Mann-Whitney U test were used to compare the distribution of continuous covariates as appropriate. OS and recurrence-free survival (RFS) curves were estimated by the Kaplan-Meier method and compared by the log-rank test. Inverse Kaplan-Meier method was used to estimate the median follow-up time. OS was calculated from the start date of the first-line chemotherapy to the date of death or last follow-up date. Time to progression was defined as time from the date of the first-line therapy to the date of first progression after the first-line therapy. Electronic medical record data was utilized to accurately capture mortality. Survival after progression was calculated from the date of first progression after the first-line chemotherapy to the date of death or last follow-up date. Univariable and multivariable Cox proportional-hazard models were fitted to assess the associations between the survival outcomes and the covariates including gender, race, Eastern Cooperative Oncology Group (ECOG) performance status (PS), insurance, best carbohydrate antigen 19-9 (CA19-9) response, and tumor differentiation. A two-tailed P value of less than 0.05 was considered to indicate statistical significance. Variables which were significant in the univariable models were added to the multivariable model. All statistical analyses were performed using R statistical package version 3.6.3 (R Core Team, Vienna, Austria).
Study objectives
Our study aims to systematically evaluate clinical outcomes for all advanced stage PDA patients treated with modern chemotherapy regimens at a single urban specialty care medical center. Our primary endpoint was OS between AA vs. White PDA patients. Secondary endpoints include OS differences between White patients and other racial groups and racial differences in cancer risk factors, clinicopathologic characteristics, receipt of systemic therapy, and systemic therapy related toxicity.
Results
Patient demographics
Out of the 145 patients who met the study criteria, 69 identified as White, 34 as AA, 15 as Asian, and 27 as Other (Figure 1, Table 1). The median age at diagnosis for the entire group was 69 years old. Asian patients were significantly younger at diagnosis (61 years old) than White patients (72 years old) (P=0.035). Approximately two thirds (68.0%) of patients who identified as Other also identified as Hispanic or Latino ethnicity (P<0.001). Most (67.6%) AA patients had public health insurance as their sole insurance provider, the largest proportion of any racial group (P=0.017). Compared to other groups, AA patients had the highest baseline BMI (P=0.004) and the largest proportion of patients whose marital status was single (P=0.008), both of which are known risk factors for PDA (5). Other risk factors such as a history of smoking, diabetes, pancreatitis and a family history of PDA were similar between racial groups (Table 1).
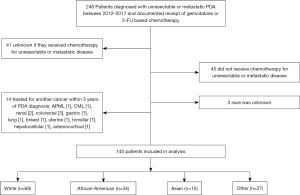
Table 1
| Variables | White | Black or African-American | Asian | Other | All subjects | P value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Number | 69 | 34 | 15 | 27 | 145 | |
| Age, median [range], years | 72 [38, 89] | 67 [45, 85] | 61 [50, 78] | 63 [39, 83] | 69 [38, 89] | 0.035 |
| Gender, n (%) | 0.094 | |||||
| Male | 47 (68.1) | 15 (44.1) | 7 (46.7) | 16 (59.3) | 85 (58.6) | |
| Female | 22 (31.9) | 19 (55.9) | 8 (53.3) | 11 (40.7) | 60 (41.4) | |
| Known ethnicity, n (%) | <0.001 | |||||
| Hispanic or Latino | 6 (8.8) | 1 (3.0) | 0 (0) | 17 (68.0) | 24 (17.0) | |
| Not Hispanic or Latino | 62 (91.2) | 32 (97.0) | 15 (100) | 8 (32.0) | 117 (83.0) | |
| Health insurance plan, n (%) | 0.017 | |||||
| Public | 30 (43.5) | 23 (67.6) | 9 (60) | 15 (55.6) | 77 (53.1) | |
| Private | 13 (18.8) | 5 (14.7) | 5 (33.3) | 9 (33.3) | 32 (22.1) | |
| Both public and private | 26 (37.7) | 6 (17.6) | 1 (6.7) | 3 (11.1) | 36 (24.8) | |
| Risk factors, n (%) | ||||||
| Known smoking history | 0.099 | |||||
| Yes | 38 (55.9) | 20 (58.8) | 3 (21.4) | 14 (51.9) | 75 (52.4) | |
| No | 30 (44.1) | 14 (41.2) | 11 (78.6) | 13 (48.1) | 68 (47.6) | |
| Diabetes history, n (%) | 0.167 | |||||
| Yes | 17 (24.6) | 15 (44.1) | 4 (26.7) | 11 (40.7) | 47 (32.4) | |
| No | 52 (75.4) | 19 (55.9) | 11 (73.3) | 16 (59.3) | 98 (67.6) | |
| Known baseline BMI, median (range), kg/m2 | 24.5 (17.6, 37.2) | 26.2 (16.3, 57.1) | 22.5 (16.7, 28.5) | 22.6 (13.7, 35.0) | 23.9 (13.7, 57.1) | 0.004 |
| Known family history of PDA, n (%) | 0.773 | |||||
| Yes | 9 (15.0) | 2 (6.9) | 1 (7.1) | 3 (11.5) | 15 (11.6) | |
| No | 51 (85.0) | 27 (93.1) | 13 (92.9) | 23 (88.5) | 114 (88.4) | |
| Known marital status, n (%) | 0.008 | |||||
| Married | 48 (70.6) | 9 (26.5) | 11 (73.3) | 13 (50.0) | 81 (56.6) | |
| Single | 11 (16.2) | 15 (44.1) | 2 (13.3) | 7 (26.9) | 35 (24.5) | |
| Widowed | 5 (7.4) | 4 (11.8) | 1 (6.7) | 2 (7.7) | 12 (8.4) | |
| Divorced | 4 (5.9) | 5 (14.7) | 1 (6.7) | 3 (11.5) | 13 (9.1) | |
| Significant other/life partner | 0 (0) | 1 (2.9) | 0 (0) | 1 (3.8) | 2 (1.4) | |
| Pancreatitis history, n (%) | 0.342 | |||||
| Yes | 2 (2.9) | 3 (8.8) | 0 (0) | 0 (0) | 5 (3.4) | |
| No | 67 (97.1) | 31 (91.2) | 15 (100) | 27 (100) | 140 (96.6) | |
BMI, body mass index; PDA, pancreatic ductal adenocarcinoma.
Therapy for earlier stage disease
Approximately one third (32.4%) of patients underwent surgery for an earlier diagnosis of localized PDA and later recurred with metastatic or unresectable disease, whereas the remaining patients had advanced PDA at diagnosis. A similar proportion of patients in each subgroup underwent resection for earlier stage disease (P=0.192) and there were no differences in receipt of neoadjuvant or adjuvant chemotherapy/chemoradiation. Median time to recurrence was 10.1 months and there were no significant differences between racial subgroups (P=0.300).
Therapy for advanced PDA
Patient characteristics at the time of therapy initiation for advanced PDA and details of treatment regimens are summarized in Tables 2,3. The vast majority (94.6%) of patients had stage IV disease, and 4.7% of patients had stage III disease. There were significant differences in baseline ECOG PS between racial groups; Asian patients had the best ECOG PS at baseline while AA patients had the worst (P=0.011). The AA group had the highest proportion of patients presenting with a baseline ECOG PS of 2 (25.8%). There were no differences in sites of metastases, baseline carcinoembryonic antigen (CEA), or baseline CA19-9 between racial groups (Table 2). Approximately one fifth of patients had known baseline CEA (15.9%) or CA19-9 (17.2%) values within normal limits.
Table 2
| Characteristics | White | African American | Asian | Other | All subjects | P value |
|---|---|---|---|---|---|---|
| Known location of metastases/recurrence, n (%) | ||||||
| Pancreas or pancreatic bed | 8 (12.7) | 5 (16.7) | 1 (7.7) | 1 (4.2) | 15 (11.5) | 0.578 |
| Liver | 40 (63.5) | 20 (66.7) | 11 (84.6) | 19 (79.2) | 90 (69.2) | 0.341 |
| Peritoneum | 12 (19.0) | 2 (6.7) | 1 (7.7) | 1 (4.2) | 16 (12.3) | 0.206 |
| Lung | 14 (22.2) | 5 (16.7) | 2 (15.4) | 8 (33.3) | 29 (22.3) | 0.492 |
| Bone | 1 (1.6) | 0 (0) | 2 (15.4) | 0 (0) | 3 (2.3) | 0.053 |
| Other | 6 (9.5) | 3 (10.0) | 2 (15.4) | 3 (12.5) | 14 (10.8) | 0.857 |
| Known baseline CEA, median (range) | 8.2 (0.4, 531.4) | 6.6 (1.0, 885.6) | 37.9 (4.8, 361.3) | 7.7 (0.9, 316.2) | 8.3 (0.4, 885.6) | 0.118 |
| Known baseline CA19-9, median (range) | 483.6 (0.8, 600,000.0) | 244.6 (0.8, 120,000.0) | 4,266.8 (1.0, 1,362,155.0) | 811.2 (1.0, 181,381.9) | 477.0 (0.8, 1,362,155.0) | 0.407 |
| Known baseline ECOG PS, n (%) | 0.011 | |||||
| 0 | 23 (41.1) | 6 (19.4) | 8 (66.7) | 7 (26.9) | 44 (35.2) | |
| 1 | 29 (51.8) | 17 (54.8) | 4 (33.3) | 18 (69.2) | 68 (54.4) | |
| 2 | 4 (7.1) | 8 (25.8) | 0 (0) | 1 (3.8) | 13 (10.4) | |
PDA, pancreatic ductal adenocarcinoma; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; ECOG PS, Eastern Cooperative Oncology Group performance status.
Table 3
| Therapy | White | African American | Asian | Other | All subjects | P value |
|---|---|---|---|---|---|---|
| 1st line systemic therapy for recurrent or metastatic PDA | ||||||
| 1st line chemotherapy regimen received, n (%) | (n=67 patients) | (n=34 patients) | (n=14 patients) | (n=27 patients) | (n=142 patients) | 0.552 |
| Gemcitabine-based chemotherapy | ||||||
| Gemcitabine | 7 (10.4) | 6 (17.6) | 0 (0) | 3 (11.1) | 16 (11.3) | |
| Gemcitabine + nab-paclitaxel | 27 (40.3) | 19 (55.9) | 6 (42.9) | 10 (37.0) | 62 (43.7) | |
| Gemcitabine + oxaliplatin | 0 (0) | 0 (0) | 0 (0) | 1 (3.7) | 1 (0.7) | |
| Fluorouracil-based chemotherapy | ||||||
| Fluorouracil + folinic acid + irinotecan | 5 (7.5) | 0 (0) | 0 (0) | 3 (11.1) | 8 (5.6) | |
| Fluorouracil + folinic acid + oxaliplatin | 4 (6.0) | 0 (0) | 0 (0) | 1 (3.7) | 5 (3.5) | |
| Fluorouracil + folinic acid + irinotecan + oxaliplatin | 17 (25.4) | 7 (20.6) | 6 (42.9) | 7 (25.9) | 37 (26.1) | |
| Capecitabine | 1 (1.5) | 0 (0) | 0 (0) | 1 (3.7) | 2 (1.4) | |
| Other chemotherapy | 2 (3.0) | 1 (2.9) | 0 (0) | 0 (0) | 3 (2.1) | |
| Investigational therapy | 4 (6.0) | 1 (2.9) | 2 (14.3) | 1 (3.7) | 8 (5.6) | |
| Known 1st line of systemic therapy duration of treatment, median (range), months | 3.2 (0, 40.7) | 3.0 (0, 18.9) | 4.3 (0.9, 9.4) | 3.1 (0, 15.6) | 3.1 (0, 40.7) | 0.863 |
| Known 1st line of course-altering chemotherapy toxicity, n (%) | (n=52 patients) | (n=27 patients) | (n=12 patients) | (n=24 patients) | (n=115 patients) | 0.396 |
| None | 19 (36.5) | 9 (33.3) | 5 (41.7) | 10 (41.7) | 43 (37.4) | |
| Neuropathy | 8 (15.4) | 3 (11.1) | 1 (8.3) | 0 (0) | 12 (10.4) | |
| GI | 12 (23.1) | 2 (7.4) | 1 (8.3) | 5 (20.8) | 20 (17.4) | |
| Hematologic | 7 (13.5) | 9 (33.3) | 2 (16.7) | 5 (20.8) | 23 (20.0) | |
| More than one (neuropathy, GI, hematologic) | 6 (11.5) | 4 (14.8) | 3 (25.0) | 4 (16.7) | 17 (14.8) | |
| Known time to progression on 1st line of systemic therapy, median (95% CI), months | 8.6 (6.2, NA) | 6.5 (5.4, 15.7) | 5.5 (3.0, NA) | 6.1 (4.0, NA) | 6.7 (5.8, 8.6) | 0.300 |
| 2nd line systemic therapy for recurrent or metastatic PDA | ||||||
| Unknown if received 2nd line of systemic therapy, n (%) | 12 (17.4) | 1 (2.9) | 1 (6.7) | 3 (11.1) | 17 (11.7) | 0.167 |
| Receipt of 2nd line of systemic therapy (for known patients), n (%) | 0.003 | |||||
| Received 2nd line therapy | 28 (47.5) | 11 (33.3) | 8 (57.1) | 13 (54.2) | 60 (46.2) | |
| Did not receive 2nd line therapy | 31 (52.5) | 22 (66.7) | 6 (42.9) | 11 (45.8) | 70 (53.8) | |
| 2nd line chemotherapy regimen received, n (%) | (n=27 patients) | (n=11 patients) | (n=8 patients) | (n=13 patients) | (n=59 patients) | 0.470 |
| Gemcitabine-based chemotherapy | 8 (29.6) | 3 (27.3) | 5 (62.5) | 6 (46.2) | 22 (37.3) | |
| Fluorouracil-based chemotherapy | 13 (48.1) | 7 (63.6) | 1 (12.5) | 3 (23.1) | 24 (40.7) | |
| Other chemotherapy | 2 (7.4) | 0 (0) | 0 (0) | 1 (7.7) | 3 (5.1) | |
| Investigational therapy | 4 (14.8) | 1 (9.1) | 2 (25.0) | 3 (23.1) | 10 (16.9) | |
| Known 2nd line of systemic therapy duration of treatment, median (range), months | 2.9 (0, 10.8) | 2.6 (0.5, 5.6) | 2.1 (0, 8.8) | 3.0 (0, 11.9) | 2.5 (0, 12) | 0.961 |
PDA, pancreatic ductal adenocarcinoma; GI, gastrointestinal.
For first-line therapy, 43.7% of patients were treated with gemcitabine + nab-paclitaxel, 26.1% received FOLFIRINOX, and 9.1% received FOLFOX or FOLFIRI. Other patients were treated with other gemcitabine or fluorouracil-based combinations or monotherapy, and 5.6% received investigational therapies. Median duration of first-line chemotherapy was 3.1 months and was similar between racial subgroups (P=0.863). Median time to progression was 6.7 months and was not significantly different between racial subgroups (P=0.300). However, there was a difference in median time to progression among patients with different metastatic sites (bone: 2.0 months, pancreas: 5.2 months, liver: 6.1 months, lung: 6.3 months, other: 10.0 months).
Approximately half (46.2%) of patients underwent second-line therapy, of whom 37.3% received gemcitabine-based regimens, 40.7% received fluorouracil-based regimens, and 16.9% received investigation therapies consisting of immune checkpoint inhibitors, Jak inhibitors, RANKL inhibitors, antiangiogenic agents, and vaccines with and without chemotherapy. There were no differences in the frequency of systemic therapy regimens received between racial groups for first- or second-line therapy (P=0.552, 0.470, respectively) (Table 3). However, AA patients were less likely to receive second-line therapy than other groups. Approximately a third of the AA cohort received second-line therapy, while approximately half of the entire patient cohort received second-line therapy (P=0.003) (Table 3). Median duration of second-line chemotherapy was 2.5 months and was not significantly different between racial groups (P=0.960).
There were 5 patients who underwent metastasectomies for ovarian, lung, liver, and splenic disease. Data on course-altering chemotherapy toxicity was also collected for first-line regimens, and there were no differences in the frequency of gastrointestinal, hematologic or neurologic toxicities between groups (P=0.396) (Table 3). Receipt of palliative radiation therapy between groups was not different (P=0.143), but AA patients most frequently received radiation therapy to the pancreas or pancreatic bed compared to distant metastases (P=0.043).
Survival outcomes
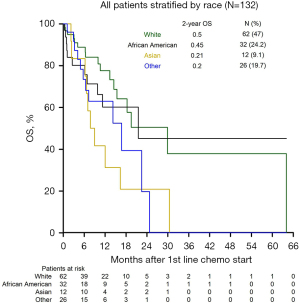
There were 50 deaths (38%), and 82 patients were lost to follow-up (62%) with complete survival data. Follow-up time ranged from 0.2 to 64.0 months, and the median follow-up time was 11.6 months. Figure 2 demonstrates OS differences between the racial groups. The median and 2-year OS for the entire group were 18 months and 39%, respectively. Median survival after progression on first-line therapy was 12.7 months and was significantly shorter in Asian patients (2.3 months), while Other patients had the longest survival after progression on first-line therapy (17.1 months). Patients who underwent surgery for earlier stage disease and then recurred had significantly better OS than patients who initially presented with unresectable or advanced disease (P=0.007).
On univariable analysis, there were no differences in OS between AA and White patients (HR 1.51, P=0.297) but Asian patients had worse OS than Whites (HR 2.74, P=0.013) (Table 4). Additionally, there were no differences in OS between Hispanic or Latino and non-Hispanic or Latino White patients nor between non-Hispanic or Latino “Other” and non-Hispanic or Latino White patients (HR 1.69, P=0.192; HR 0.84, P=0.816, respectively) (Table 4). Receipt of gemcitabine-based regimens first line was associated with worse OS compared to fluorouracil-based regimens (HR 2.43, P=0.008) (Table 4), but there was no significant difference in OS between patients who received FOLFIRINOX vs. gemcitabine + nab-paclitaxel in first line (P=0.110). There was also no significant association between OS and number of metastatic sites (HR 0.91, P=0.696), best CA19-9 response (<50% vs. ≥50% decrease, HR 1.28, P=0.473), or tumor differentiation (poor vs. well/moderate HR 1.07, P=0.871). The survival disadvantage for Asian compared to White patients (HR 2.74, P=0.013) and receipt of gemcitabine-based regimens (HR 2.43, P=0.008) (Table 4) in first line were confirmed on multivariable analysis.
Table 4
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Univariable model results | |||
| Ethnicity | |||
| Hispanic or Latino vs. not Hispanic or Latino | 1.38 | 0.69, 2.78 | 0.366 |
| Race and ethnicity | |||
| African American vs. White | 1.36 | 0.63, 2.93 | 0.437 |
| Asian vs. White | 2.48 | 1.12, 5.48 | 0.025 |
| Hispanic or Latino vs. White | 1.69 | 0.77, 3.74 | 0.192 |
| Other vs. White | 0.84 | 0.19, 3.67 | 0.816 |
| Gender | |||
| Male vs. female | 1.43 | 0.8, 2.57 | 0.228 |
| Race | |||
| African American vs. White | 1.51 | 0.7, 3.25 | 0.297 |
| Asian vs. White | 2.74 | 1.24, 6.05 | 0.013 |
| Other vs. White | 2.05 | 0.96, 4.36 | 0.062 |
| ECOG PS | |||
| 1 vs. 0 | 0.86 | 0.46, 1.62 | 0.647 |
| 2 vs. 0 | 1.82 | 0.68, 4.88 | 0.233 |
| CA19-9 | |||
| Every 10-fold increase in CA19-9 | 1.16 | 0.92, 1.45 | 0.211 |
| Insurance type | |||
| Both vs. private | 0.59 | 0.26, 1.31 | 0.192 |
| Public vs. private | 0.88 | 0.45, 1.72 | 0.707 |
| 1st line chemotherapy type | |||
| Gemcitabine vs. fluoropyrimidine-based regimens | 2.43 | 1.26, 4.69 | 0.008 |
| Number of metastatic sites | |||
| Every unit increase | 0.91 | 0.55, 1.49 | 0.696 |
| Best CA19-9 response | |||
| <50% vs. ≥50% decrease | 1.28 | 0.65, 1.52 | 0.473 |
| Tumor differentiation | |||
| Poor vs. well/moderate | 1.07 | 0.45,2.54 | 0.871 |
| Multivariable model results | |||
| Race and ethnicity | |||
| African American vs. White | 1.12 | 0.52, 2.45 | 0.769 |
| Asian vs. White | 2.62 | 1.18, 5.82 | 0.018 |
| Hispanic or Latino vs. White | 1.49 | 0.66, 3.38 | 0.340 |
| Other vs. White | 0.80 | 0.18, 3.50 | 0.763 |
| 1st line chemotherapy type | |||
| Gemcitabine vs. fluoropyrimidine-based regimens | 2.65 | 1.34, 5.23 | 0.005 |
ECOG PS, Eastern Cooperative Oncology Group performance status; CA19-9, carbohydrate antigen 19-9.
Discussion
This study was motivated by an apparent epidemiological racial disparity in PDA incidence with regards to age and stage at presentation, treatment and survival. Our study systematically evaluated clinical outcomes for all advanced stage PDA patients treated with modern chemotherapy regimens at a single urban specialty care medical center. Previous studies have reported that early-stage AA patients may be less likely to receive surgery and/or chemotherapy, which adversely impacts OS for those patients (7,9). Notably, we compared whether there were disparities in receipt of specific first- and second-line treatment regimens, duration of treatment, and course-altering toxicities between racial groups—a level of depth not reported in previous epidemiological studies. This level of depth provided by our retrospective review at a single institution contributes to the existing literature by providing insight into the contributions of specific clinical data on PDA OS disparities that complements prior epidemiological reports.
Although we expected to observe OS disparities between AA and White patients based on previous reports in the literature, the poorer outcomes in Asian patients were an unexpected finding. To our knowledge, this study is the first to report a survival disadvantage for advanced stage Asian PDA patients compared to White patients, though it must be noted that only 15 Asian patients were included, and this finding will require confirmation in larger studies. In a study of Surveillance, Epidemiology, and End Results (SEER) data from Hawaii, San Francisco, and Seattle that included 1,340 patients of Asian descent, outcomes differed between subgroups of Asian patients (4). Patients of Japanese descent were most frequently diagnosed with localized disease, while patients of Hawaiian descent were more frequently diagnosed at a younger age and with metastatic disease. However, the same study reported no differences in survival between Asians, AA and White patients. The molecular pathogenesis of PDA may also differ in Asian patients. A recent report found that Asian patients might be more likely to have specific single nucleotide polymorphisms of the ERCC2 gene, which may confer an enhanced propensity for pancreatic carcinogenesis (13). Additionally, a study of 59 Chinese PDA patients reported differences in KRAS point mutations and p53 co-expression compared to Western patients (14). It is possible that Asian patients might suffer from more biologically aggressive tumors that lead to inferior OS. However, further analyses with larger sample sizes including different subgroups of Asian populations may help better characterize the presence of a PDA survival disparity for a particular group of Asian patients and provide insight into possible contributing factors.
The 2-year survival rate of 39% in this population of heterogeneously treated patients with advanced PDA was another unexpected finding. In the phase III studies of first-line FOLFIRINOX and gemcitabine + nab-paclitaxel for advanced PDA, 2-year OS was approximately 10% (15,16). Median duration of first-line therapy was shorter in our cohort (3.1 months) than in the FOLFIRINOX trial (~5 months), but time to progression (6.7 months) was comparable to progression-free survival reported with FOLFIRINOX (6.4 months) and gemcitabine + nab-paclitaxel (5.3 months). Furthermore, a similar proportion of patients in all studies received 2nd line therapy. A comparison of our study cohort with the subjects enrolled in both trials revealed similarities in the median age, male:female ratio, and PS distribution. However, there was a lower frequency of liver metastases in our cohort (69.2%) compared to both phase III trials (84–88%), and 12.3% of our patients had peritoneal disease compared to ~19% of patients in the FOLFIRINOX trial. Additionally, the percentage of patients who underwent a prior resection was not reported by Conroy et al., but comprised 7% of patients in the gemcitabine + nab-paclitaxel trial compared to 32.4% of patients included in the current study. It is possible that there is a difference in tumor biology between patients presenting with metastatic disease at diagnosis versus those recurring after resection, and the inclusion of a larger proportion from the latter cohort in our study likely contributed to the observed difference in OS. In our cohort, median survival for patients presenting with metastatic disease was inferior to patients presenting with recurrent disease (HR 2.63, P=0.009, data not presented). The lower frequency of some poor risk characteristics in our patient population might have contributed to the survival discrepancy. Additionally, it is likely that our retrospective study suffers from survivor bias that limits comparisons with the data presented in the prospective phase III randomized control trials.
Given the retrospective nature of our study, there are inherent shortcomings that should be taken into account when considering the external validity of our findings. Though there were only 145 patients who met our criteria and groups were not equally represented, the racial composition of our cohort is reflective of our institution’s neighboring population according to the 2010 census data. In our study, 48% of the patients were White, 23% of the patients were AA, 10% were Asian, and 17% were Hispanic or Latino, while the 2010 US Census documented the following distribution of racial groups in New York City (45% White, 25% AA, 12% Asian, and 28% Hispanic or Latino). Race was self-reported in our electronic medical record data and not based on genetic ancestry. The composition of racial groups in our study is consistent with the self-reported US Census data, which supports the notion that our sample is representative of our institution’s patient population. There were missing baseline risk factors, disease characteristics, and treatment data that could not be retrieved retrospectively in the medical charts, and for these fields the “Known” data was reported. A significant number of patients were lost to follow up and appropriate statistical assumptions were incorporated into our survival models. To demonstrate this limitation, Figure 2 incorporates the “patients at risk”, providing an accurate visual representation of the number of patients lost to follow up at each timepoint for each group. Finally, the retrospective nature of this study also created limitations in statistical power, which precluded our ability to include all variables of interest into univariable and multivariable analyses.
Future investigation of potential socioeconomic contributions to the survival disparity observed in our study is needed. While insurance status was used a proxy for socioeconomic status in our study and included in our OS model, it was not found to impact OS. Given the limitations of the retrospective nature of our study, additional socioeconomic data was difficult to collect and account for. Some specific additional factors that would be useful for future studies include the investigation of the impact of ZIP code, income level, and education level, among others on PDA OS (17).
Additionally, tumor biology could be important to investigate in future PDA disparities studies. It has been reported that the somatic mutational profile might be different between races, specifically with respect to KRAS mutational status (14,18). Next generation sequencing data was available for 30 patients (~20%), and 6 patients (~4%) had germline molecular data available. The limitations associated with the retrospective nature of this report precluded our ability to perform statistical comparisons with molecular data between groups. The presence of a differential somatic mutation burden between racial groups would likely reflect a differential cancer risk status influenced by environmental socio-economic risk factors that impose selective oncogenic pressures, rather than differences in cancer genetic predisposition at baseline. Furthermore, it is imperative to proceed with caution in any investigation that categorizes patients by race, specifically with implications of tumor biology and cancer predisposition. Every effort should be made to ensure that the research subjects’ racial identity is self-selected when using race as a variable, and any reports on biology and genetics should use ancestry linkage if possible, as race is a social construct confounded by sociocultural and political factors (19).
In this retrospective series of advanced stage PDA patients treated with contemporary chemotherapy, AA and Whites had comparable outcomes, but Asians had worse OS than Whites. Further study of socioeconomic contributors to this health disparity along with possible disparities in tumor biology is warranted.
Acknowledgments
Funding: This work is supported by the resources and facilities in the Division of Hematology/Oncology in the Department of Medicine at the Icahn School of Medicine at Mount Sinai in New York, New York. Research reported in this publication was supported in part by the National Cancer Institute Cancer Center Support Grant P30CA196521-01 awarded to the Tisch Cancer Institute of the Icahn School of Medicine at Mount Sinai and used by the Biostatistics Shared Resource Facility. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the Medical Student Research Office at the Icahn School of Medicine at Mount Sinai.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apc-21-5
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apc-21-5
Peer Review File: Available at https://dx.doi.org/10.21037/apc-21-5
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apc-21-5). Dr. CA received the following over the past 36 months: investigator initiated pilot study seed grant ($50,000) awarded by the Tisch Cancer Institute at Mount Sinai, award period 7/2019–7/2020; travel reimbursement by the Tisch Cancer Institute for conference attendance. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (NO. 19-01539) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Khawja SN, Mohammed S, Silberfein EJ, et al. Pancreatic cancer disparities in African Americans. Pancreas 2015;44:522-7. [Crossref] [PubMed]
- Fitzgerald TL, Bradley CJ, Dahman B, et al. Gastrointestinal malignancies: when does race matter? J Am Coll Surg 2009;209:645-52. [Crossref] [PubMed]
- Longnecker DS, Karagas MR, Tosteson TD, et al. Racial differences in pancreatic cancer: comparison of survival and histologic types of pancreatic carcinoma in Asians, blacks, and whites in the United States. Pancreas 2000;21:338-43. [Crossref] [PubMed]
- Silverman DT, Hoover RN, Brown LM, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology 2003;14:45-54. [Crossref] [PubMed]
- Arnold LD, Patel AV, Yan Y, et al. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity? Cancer Epidemiol Biomarkers Prev 2009;18:2397-405. [Crossref] [PubMed]
- Nipp R, Tramontano AC, Kong CY, et al. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med 2018;7:525-35. [Crossref] [PubMed]
- Tohme S, Kaltenmeier C, Bou-Samra P, et al. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Ann Surg Oncol 2018;25:3427-35. [Crossref] [PubMed]
- Heller DR, Nicolson NG, Ahuja N, et al. Association of Treatment Inequity and Ancestry With Pancreatic Ductal Adenocarcinoma Survival. JAMA Surg 2020;155:e195047 [Crossref] [PubMed]
- Abraham A, Al-Refaie WB, Parsons HM, et al. Disparities in pancreas cancer care. Ann Surg Oncol 2013;20:2078-87. [Crossref] [PubMed]
- Wray CJ, Castro-Echeverry E, Silberfein EJ, et al. A multi-institutional study of pancreatic cancer in Harris County, Texas: race predicts treatment and survival. Ann Surg Oncol 2012;19:2776-81. [Crossref] [PubMed]
- Singal V, Singal AK, Kuo YF. Racial disparities in treatment for pancreatic cancer and impact on survival: a population-based analysis. J Cancer Res Clin Oncol 2012;138:715-22. [Crossref] [PubMed]
- Wu Y, Lu ZP, Zhang JJ, et al. Association between ERCC2 Lys751Gln polymorphism and the risk of pancreatic cancer, especially among Asians: evidence from a meta-analysis. Oncotarget 2017;8:50124-32. [Crossref] [PubMed]
- Dong M, Nio Y, Tamura K, et al. Ki-ras point mutation and p53 expression in human pancreatic cancer: a comparative study among Chinese, Japanese, and Western patients. Cancer Epidemiol Biomarkers Prev 2000;9:279-84. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 2006;17:5-19. [Crossref] [PubMed]
- Pernick NL, Sarkar FH, Philip PA, et al. Clinicopathologic analysis of pancreatic adenocarcinoma in African Americans and Caucasians. Pancreas 2003;26:28-32. [Crossref] [PubMed]
- Kittles RA, Weiss KM. Race, ancestry, and genes: implications for defining disease risk. Annu Rev Genomics Hum Genet 2003;4:33-67. [Crossref] [PubMed]
Cite this article as: Williams M, Özbek U, Lin JY, Ang C. A single-institutional analysis of racial disparities in clinicopathologic characteristics, treatment selections, and outcomes in advanced-stage pancreatic cancer patients. Ann Pancreat Cancer 2021;4:7.