
Selected pre-operative factors which affect pancreaticoduodenectomy outcomes: a systematic review
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fifth biggest cancer killer in the United Kingdom (UK); five-year survival is around 7% (1). For patients with early disease affecting the head of the pancreas, the only treatment option which provides the possibility of long-term survival is surgical resection in the form of pancreaticoduodenectomy (PD). Despite improvements to surgical technique, patient selection and peri-operative care, morbidity remains high and most patients develop recurrent disease. This review aims to consolidate the recent literature on pre-selected pre-operative factors which affect peri-operative and survival outcomes following PD performed for suspected PDAC. The factors selected are currently being investigated by the Recurrence After Whipple’s (RAW) study (https://clinicaltrials.gov/ct2/show/NCT04596865). An appreciation of these will guide patient selection, pre-operative optimisation, and risk/benefit discussions with potential surgical candidates. Data on these factors will also allow for the development of predictive models so that the likelihood of certain outcomes can be estimated in individual patients. We present the following article in accordance with the PRISMA checklist (available at https://dx.doi.org/10.21037/apc-21-15).
Methods
The pre-operative factors included were all selected prior to carrying out the literature search. These were: age, gender, body mass index (BMI), sarcopenia, myosteatosis, diabetes mellitus (DM), cardiac disease, respiratory disease, radiological tumour characteristics, neoadjuvant treatment (NAT), biliary stenting, bilirubin, C-reactive protein (CRP), albumin, C-reactive protein/albumin ratio (CAR) and neutrophil/lymphocyte ratio (NLR). A systematic search of the English literature was carried out on 1st June 2021. The PubMed database were searched using the terms “pre-operative factor in question”, “pancreaticoduodenectomy”, and “outcome” from May 2011 through May 2021. The following articles were included: (I) human studies; (II) English language; (III) meta-analyses (MA), systematic reviews (SR) or clinical studies reporting on peri-operative outcomes and survival following open PD performed for suspected PDAC; (IV) excluding the radiology and NAT sections, minimum of 100 PDs (if final histological diagnosis specified, at least 100 PDs performed for PDAC); (V) in terms of risk factors/associations, only statistically significant results were included (P<0.05); (VI) to reduce the impact of bias, studies were only included if the “pre-operative factor in question” was investigated as a primary outcome measure and did not depend on other factors. For the radiological features section, a non-systematic search was undertaken (not using the stated criteria) to identify articles reporting on specific radiological features which affect PD outcomes. Concerning NAT, only articles reporting on comparisons between NAT and standard of care (upfront surgery) were included, and those comparing different NAT regimens were excluded. For the biliary stenting section, only studies comparing stenting to upfront surgery were included, and studies comparing stenting methods or timing of PBS were excluded.
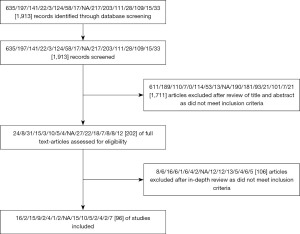
The initial search returned 1,913 records (Figure 1). After initial screening, 1,711 articles were excluded as they did not meet the inclusion criteria. Following an in-depth review of the remaining articles, a further 106 were excluded. Ninety-six articles were included in the final analysis. Eleven of these were SRs/MAs and the remainder were single/multi-centre studies. Figure 1 illustrates the breakdown. No amendments were made to the original methods.
Results
Age
Median age at PDAC diagnosis is 70 years and the average age of patients presenting with resectable disease is set to rise (2). Whilst decisions to operate must never be based solely on numerical age, a pragmatic and patient-centred approach should be employed. Multiple recent studies have concluded that it is safe and reasonable to perform PD in selected older patients. Shamali et al. (3) (n=524) showed that patients aged ≥75 years had similar rates of overall morbidity and major morbidity compared to younger patients. Furthermore, age was not an independent predictor of five-year or overall survival (OS) (3). However, the older patients were more likely to experience cardiac complications (10.8% vs. 3.6%, P=0.008) and had higher peri-operative mortality (5.9% vs. 1.9%, P=0.037). In contrast, El Nakeeb et al. (4) (n=828) found patients aged >70 years had the highest overall morbidity, followed by those aged 60–70 years, followed by under 60s (25.9% vs. 36.8% vs. 37.5%, P=0.006). However, peri-operative mortality rates were similar (4). Zhang et al. (5) (n=216) reached similar conclusions. Patients >70 years had similar morbidity and mortality rates to those ≤70, but were more likely to experience cardiac (P=0.008) or respiratory (P=0.013) complications, and had longer length of stay (P=0.013). Similarly, Wiltberger et al. (6) (n=370) found that age did not affect overall mortality, but that increasing age was associated with major morbidity (P<0.05).
Gruppo et al. (7) (n=106) found that being aged >70 years did not affect overall morbidity, peri-operative mortality, or OS. Other authors have reached similar conclusions using thresholds of 75 Jeny(8-10) and 80 years (2,11). In contrast, Oguro et al. (12) (n=561, 13 vs. 82 months, P=0.014) and Kim et al. (13) (n=165, 16.6 vs. 22.5 months, P=0.048) found OS was shorter in those aged >80 years.
The studies discussed will have been influenced by selection bias as older patients will have been pre-assessed as suitable surgical candidates based on their performance status and pre-existing co-morbidities. Hence, the effect of increasing age is likely underestimated. A recent SR by Kim et al. (14) (18 studies, n=49,449) concluded that over 80s have a 50% increased risk of peri-operative morbidity and a 100% increased risk of peri-operative mortality compared to under 80s. Haigh et al. (15) (n=2,610), also found that over 70s had higher rates of morbidity (40.7% vs. 34.0%, P=0.01) and mortality (4.7% vs. 1.3%, P=0.01). Further authors have reached similar conclusions using thresholds of 75 (16) and 80 years (17). As such, careful patient selection is required when deciding to operate on the elderly, but advanced age alone is not an absolute contraindication to PD.
Gender
No recent studies have specifically compared outcomes in males and females. Williamsson et al. (18) investigated for gender differences in treatment and outcomes following a diagnosis of a pancreatic head malignancy. All patients in the Swedish national database (2012–2017) were included (n=5,677, 4,227 of which had pancreatic cancer). Females were significantly older than males at time of diagnosis (72 vs. 70 years, P<0.001) and a lower proportion underwent curative-intent surgery (41 vs. 44, P=0.008). However, once age and tumour location were adjusted for, no difference was observed (18). Females had shorter operation times (376 vs. 402 min, P<0.001) and reduced intra-operative blood loss (400 vs. 600 mL, P<0.001), which may be because men tend to have a higher proportion of intra-abdominal fat (18). No difference in overall morbidity, length of stay or peri-operative mortality was observed (18). Five-year survival following resection was significantly higher in females (8.1% vs. 5.7%, P=0.046) (18). Hence, the authors concluded that it may be reasonable to offer females PD at a more advanced age (18).
Mazmudar et al. (19) (n=22,086) found, after adjusting for confounding factors, males were more likely to have an operation lasting more than 6 h (28.0% vs. 18.3%), and had higher intra-operative blood transfusion rates (14.4% vs. 14.0%), higher surgical site infection (SSI) rates (20.4% vs. 17.1%) and longer length of stay (9.4 vs. 9.1 days, all P<0.001). Again, the authors suggested that this may be the result of higher rates of abdominal-type obesity among males (19). Male sex was not associated with increased peri-operative mortality, and long-term outcomes were not studied (19).
BMI
Numerous studies have concluded that patients of an unhealthy weight are at increased risk of morbidity. The threshold BMI used varies considerably between studies. Chen et al. (20) (n=362) concluded that BMI >24 kg/m2 was associated with increased morbidity (42.9% vs. 29.6%, P=0.009), but not mortality. Aoki et al. (21) found that BMI >25 kg/m2 was a risk factor for grade C postoperative pancreatic fistula (POPF; OR =1.8) and major morbidity (OR =1.84; both P<0.001). Tang et al. (22) (n=227) reached similar conclusions. El Nakeeb et al. (23) (n=471) found that BMI >25 kg/m2 was associated with longer operation times (5.35 vs. 5.0 h, P=0.003), POPF (25.0% vs. 8.1%, P<0.001), overall morbidity (33.0% vs. 17.3%, P=0.001) and peri-operative mortality (7.1% vs. 0.8%, P=0.001). Del Chiaro et al. (24) (n=367) also found BMI >25 kg/m2 was associated with increased intra-operative blood loss (1,392 vs. 1,121 mL, P=0.01) and risk of POPF (20.0% vs. 9.5%, P=0.006), and Greenblatt et al. (25) (n=4,945) concluded that BMI >25 kg/m2 was a predictor of overall morbidity (P<0.05), but not peri-operative mortality. A recent SR and MA by You et al. (26) (22 studies, n=8,994) compared high BMI (>25 kg/m2) to low BMI (<25 kg/m2). High BMI was associated with increased operation time (mean increase: 15 min), increased intra-operative blood loss (mean difference: 271 mL), POPF (OR =1.96), delayed gastric emptying (DGE; OR =1.62), SSI (OR =1.43), and longer length of stay (mean difference: 2.87 days; all P<0.05) (26).
Using a threshold BMI of 30 kg/m2, Wiltberger et al. (6) (n=405) concluded that obese patients were more likely to experience major morbidity (P=0.05). Similarly, Ekström et al. (27) (n=328) found that obesity was associated with increased major morbidity (OR =1.72; P=0.001) and grade B/C POPF (OR =4.16; P=0.001). Using the same threshold, Chang et al. (28) (n=3,484), concluded that obesity was associated with increased rates of SSI (OR =1.38; P=0.01), unplanned return to theatre (OR =1.39; P<0.05), failure to extubate after 48 h (OR =1.6; P=0.02), septic shock (OR =2.2; P=0.0002), and peri-operative mortality (OR =1.7; P<0.05).
Zorbas et al. (29) (n=2,667), found that severe (or morbid) obesity (BMI ≥40 kg/m2) was a risk factor for pulmonary embolism (2.2% vs. 0.9%, P=0.048), POPF (30.4% vs. 16.1%, P<0.0005), SSI (15.2% vs. 8.9%, P<0.0005), renal failure (3.3% vs. 0.4%, P=0.003), and overall morbidity (65.2% vs. 47.8%, P<0.001), but not peri-operative mortality.
The increased risks associated with obesity are well documented but being underweight also has associated risks. Pausch et al. (30) (n=408) found that patients with BMI <18.5 kg/m2 had higher peri-operative mortality (P<0.048). However, this included just 16 patients in the underweight category and these findings have not been validated by larger studies. It is likely that it is malnutrition and cachexia, rather than low BMI alone, which contributes to adverse outcome.
Whilst many studies have investigated the impact of BMI on short-term outcomes, few have considered long-term outcomes. Tsai et al. (31) (n=795) concluded that overweight (BMI ≥25 kg/m2) and obese (BMI ≥30 kg/m2) patients had improved five-year survival versus normal weight patients (22% vs. 22% vs. 15%, P=0.02). Two similar studies did not observe this (32,33).
Sarcopenia
Sarcopenia is a syndrome which results in the progressive loss of skeletal muscle quality and mass, and a low level of physical performance; definitions vary between sources (34). Sarcopenia can be evaluated by assessing psoas mass and density on abdominal computed tomography (CT) at the level of the third lumbar vertebra (35). Numerous recent studies have investigated the impact of CT changes associated with sarcopenia on PD outcomes. Linder et al. (36) (n=139) found an association between pre-operative sarcopenia and severe POPF (OR =4.3; P=0.03). Several other authors have arrived at the same conclusion (37-40). Takagi et al. (41) (n=219) showed sarcopenic patients had higher rates of infective complications (67.2% vs. 40.2%, P<0.001) and peri-operative mortality (5.5% vs. 0.0%, P=0.004).
Concerning long-term outcomes, Ryu et al. (40) (n=252) found that pre-operative sarcopenia was associated with decreased five-year survival (23.4% vs. 28.4%, P=0.046). An association was also demonstrated between sarcopenic obesity and POPF (P=0.018) (40). Stretch et al. (42) (n=123) also found that sarcopenic patients had reduced OS (16.0 vs. 26.4 months, P=0.005). Peng et al. (43) (n=116) and Gruber et al. (44) (n=133) reached similar conclusions. The latter also showed that patients with sarcopenic obesity had even worse OS (14 vs. 23 months, P=0.007) and higher major morbidity rates (13.5% vs. 1.5%, P<0.001) than sarcopenic patients of a healthy weight (44).
Myosteatosis
Myosteatosis refers to fat deposition within the muscles; it can be assessed using CT or magnetic resonance imaging (MRI) where it appears as low skeletal muscle radiation attenuation. Although few studies have investigated the impact of myosteatosis on long-term outcomes of PD, Stretch et al. (42) (n=123) concluded that myosteatosis was associated with reduced OS, but only when in combination with sarcopenia (P=0.002). Only a trend was observed in myosteatosis patients without sarcopenia (P=0.06). Similarly, few studies have investigated the impact of pre-operative myosteatosis on peri-operative outcomes. However, there is recent evidence to suggest an association with increased morbidity following resection for oesophageal and gastric cancers (45). West et al. (46) (n=123) prospectively studied patients undergoing hepatobiliary and pancreatic surgery (all resections) and found that myosteatosis on pre-operative CT was associated with worse pre-operative fitness as measured by cardiopulmonary exercise testing (CPET) (P<0.001). The authors concluded that combining myosteatosis and physical fitness variables may be useful for stratifying risk (46). One would expect patients with myosteatosis to have worse peri-operative outcomes, but this remains unproven. Furthermore, it is unknown if optimising patients with myosteatosis would be of benefit.
DM
The impact of DM on outcomes following PD remains controversial. Lv et al. (47) carried out a MA (17 observational studies, n=5,407 patients, all forms of pancreatic resection included) and found that diabetic patients had higher prevalence of male sex (P=0.01) and higher BMI (P<0.001). No differences were observed in age, smoking status, prevalence of jaundice, operation time, or rate of intra-operative blood transfusion (47). Histologically, DM patients were more likely to have poorly differentiated (P=0.03), larger tumours (P<0.001), and “hard” pancreas consistency (P<0.001) (47). Cancer stage and margin status were comparable between the two groups (47). The authors, like Nakata et al. (48) in another SR, did not find DM affected overall morbidity or perioperative mortality (47,48).
POPF is a significant and well-documented complication of pancreatic resection which has been associated with DM since diabetics are thought to have a softer pancreas due to higher fat content. Small calibre pancreatic duct and soft pancreas consistency are known predisposing factors. Lv et al. (47) and Xia et al. (49) (MA of 16 studies) found similar prevalence of small pancreatic duct and soft pancreas consistency among diabetics and non-diabetics. No association between DM and POPF was observed (47,49). This may be accounted for by patient selection and the high levels of attention which are often given to high-risk patients. Another complication often linked with DM is DGE. In contrast to a few small case series, no large studies have suggested that diabetics are at increased risk of DGE.
Long-term hyperglycaemia is known to impair immune function. Hence, DM is often presumed to increase the risk of infective complications. King et al. (50) concluded that poorly controlled diabetics are more likely to experience infective complications when undergoing general and vascular surgery. Whilst the underlying mechanisms are not well understood, it is thought hyperglycaemia can affect chemotaxis, the activation of macrophages, pathogen opsonisation, and phagocytosis (51). However, the MA by Lv et al. did not identify DM as a predictor of infective complications (47). This study did show that a recent diagnosis of DM (within two years of resection) was associated with reduced OS following PD (RR =1.35; P<0.001) (47).
Cardiac disease
The impact of acute and chronic cardiac disease on pancreatic resection outcomes was investigated by Ronnekleiv-Kelly et al. (52) in a large retrospective cohort study using USA national data (n=13,021, 2/3 underwent PD). Patients were categorised as having a history of cardiac disease if they had a prior diagnosis of congestive cardiac failure (CCF), angina, or myocardial infarction (MI), or if they had any history of percutaneous coronary intervention or cardiac surgery. Eleven percent of patients had pre-existing cardiac disease and a 1.1% sub-set had “acute cardiac disease” (defined as CCF symptoms within 30 days, angina within 1 month, or MI within 6 months of surgery). Those with cardiac disease were older, more co-morbid, more likely to be male, and were more likely to experience cardiac complications (all P<0.001). Patients with acute cardiac disease were at even higher risk of cardiac complications (P<0.001) (52). A history of cardiac disease and acute cardiac disease were associated with a 1.6- (P<0.0001) and 1.8-fold (P<0.0007) increase in major morbidity, and a 2.3- (P<0.0001) and 4.2-fold (P<0.0001) increase in peri-operative mortality, respectively (52). Other studies which did not specifically investigate the impact of pre-existing cardiac disease have come to similar conclusions (25,53,54). It is unknown whether pre-existing cardiac disease affects long-term PD outcomes.
Respiratory disease
It is important to identify patients with pre-existing respiratory disease and optimise their functional status wherever possible. It is also important that patients are risk-stratified and that, as with cardiac disease, their increased level of risk is discussed with them. Pre-operative CPET can provide estimates of aerobic and anaerobic threshold to aid in pre-operative planning for the peri-operative period. Few large studies have specifically investigated the impact of pre-operative respiratory co-morbidities on PD outcomes. This is likely because those with significant respiratory disease are unlikely to be considered surgical candidates. Shia et al. (55) (n=8,490) found pre-existing chronic obstructive pulmonary disease independently reduced 90-day survival (aHR =1.35; P<0.001) and Aoki et al. (21) (n=17,564) found those with pre-existing respiratory co-morbidities had higher major morbidity (OR =1.86; P=0.012) and grade C POPF (OR =2.08; P=0.0002) rates.
Radiological features
To our knowledge, no studies have specifically investigated the impact of radiological stage on PD outcomes. One would assume that more advanced stage is associated with worse short- and long-term outcomes. Several recent studies have attempted to identify radiologic features as prognostic predictors. Lee et al. (56) (n=143) studied patients who underwent MRI within one month of PD and were subsequently found to have an R0 resection. Rim-enhancement at dynamic contrast material-enhanced MRI was associated with reduced three-year DFS (8.0% vs. 24.3%, P=0.008) and three-year OS (19.7% vs. 41.0%, P=0.001). Rim-enhancing lesions were also associated with more aggressive tumours on pathologic staging (P=0.002) (56). Several studies have investigated CT tumour characteristics. Kim et al. (57) (n=116) found tumours with a heterogeneous texture were associated with reduced DFS (6.72 vs. 10.52 months, P=0.025) and Zhu et al. (58) (n=79) that lower relative enhancement change was associated with shorter DFS (10.7 vs. 17.9 months, P=0.01) and three-year OS (20.3 vs. 28.5 months, P=0.01). Cassinotto et al. (59) (n=99) studied the portal venous phase of pre-operative scans and concluded that hypoattenuating tumours were associated with reduced one-year DFS (35.0% vs. 68.0%, P=0.04).
Positron emission tomography (PET)-CT is a further imaging modality which has been studied. Choi et al. (60) (n=64) found patients with a tumour with a maximum standardised uptake value >3.5 had reduced DFS (9.2 vs. 26.1 months, P=0.002) and OS (23.5 vs. 45.4 months, P=0.002). Yamamoto et al. (61), who performed a similar study but used a cut-off value of 6.0, came to the same conclusion. Lee et al. (62) (n=87) identified both metabolic tumour volume and total lesion glycolysis as independent predictors of DFS (HR =2.34, P=0.001; HR =2.59, P=0.003) and OS (HR =3.69, P=0.02; HR =4.85, P=0.003).
NAT
NAT aims to treat micrometastases, downstage primary tumours, and increase the chance of patients completing a course of treatment. Currently, UK national guidelines only advise NAT in PDAC patients as part of a clinical trial (63). The use of neoadjuvant chemotherapy (NAC) for resectable/borderline resectable PDAC remains a source of debate and has been the subject of several recent trials. The two-arm randomised phase II/III Prep02/JSAP05 trial involved 57 Japanese centres. One arm received gemcitabine and S-1 prior to surgery, and the other had upfront surgery. All patients with resectable or borderline resectable PDAC who could tolerate curative-intent surgery were included (n=362). OS was significantly longer in the NAC arm (36.7 vs. 26.6 months, P=0.015) (64). No difference was observed in terms of resection rate, R0 resection rate, and overall morbidity (64). The international phase II ESPAC-5F trial contained four arms. This aimed to compare resection rates in those who underwent upfront surgery to gemcitabine/capecitabine NAC, FOLFIRINOX (folinic acid, fluorouracil, irinotecan and oxaliplatin-based) NAC, and neoadjuvant chemo-radiotherapy (NACRT) (n=90). Resection rate was slightly higher in the upfront surgery group, but this was not significant (65). Upfront surgery was associated with reduced one-year survival compared to all NAT arms (40% vs. 77%, P<0.001); the authors concluded that NAT should be considered in those with borderline resectable PDAC (65).
The phase III PREOPANC trial involved 16 Dutch centres and aimed to compare outcomes in those who received NACRT to those who received conventional treatment (upfront surgery followed by gemcitabine-based adjuvant chemotherapy) (n=248). All surgical candidates with pathologically confirmed PDAC with resectable or borderline resectable disease were included. T1 tumours were excluded, and randomisation took place prior to biliary drainage. Those in the NACRT arm had a slight survival benefit although this was not significant (66). When those in the NACRT group who failed to progress to surgery were excluded, R0 resection rate was significantly higher in the NACRT group compared to the upfront surgery group (71% vs. 40%, P<0.001). Hence, NACRT likely improved the process of selecting surgical candidates (66). When only those who underwent resection and subsequently started adjuvant therapy were included, NACRT provided a further survival benefit (35.2 vs. 19.8 months, P=0.029) (66).
A recent MA by Rangarajan et al. (67) included 27 studies: three randomised controlled trials (RCTs) and 24 retrospective cohort studies (n=63,151). Improved survival outcomes (HR =0.72; P<0.001), reduced morbidity rates (RR =0.81; P=0.001) and improved R0 resection rates (RR =0.51; P<0.001) were observed in those who received NAC. Greco et al. (68) (n=8,472) reached similar conclusions. These studies will have been affected by selection bias since patients who received NAC but failed to progress to surgery were excluded. Both authors concluded that, whilst there may not be strong evidence for NAC in resectable disease, it does confer a survival benefit for select patients and that randomised trials are needed (67). In a further MA by Lee et al. (69) (14 studies, n=9,691), NAC was not found to provide a survival benefit. However, patients who received NAC showed improved OS when compared with patients who had upfront surgery and then completed adjuvant treatment (HR =0.82; P<0.001) (69). The authors concluded that, whilst NAC may not provide an obvious survival benefit for all patients, it may have a role in selecting suitable candidates for resection (69).
Whilst the survival benefits of NAT continue to be investigated, it is important to consider whether NAT affects peri-operative outcomes. Kamarajah et al. (70) (n=7,975) found that patients receiving NAT had lower rates of unplanned readmission (5.5% vs. 7.4%, P=0.006) and that NAT had no effect on length of stay or peri-operative mortality. Cho et al. (71) (n=4,416) found patients who received NAT had longer operation times (423 vs. 368 min, P<0.001) and were more likely to undergo vascular reconstruction (20.5% vs. 8.4%, P<0.001). This is likely because patients who underwent NAT were more likely to have named vessel involvement as their indication for chemotherapy. No difference was observed in morbidity or mortality rates, and those in the NAT group had shorter length of stay (9 vs. 10 days, P=0.005) (71). In a similar study, Cools et al. (72) (n=3,748) found NAT patients were more likely to undergo named vein resection (35.8% vs. 17.6%, P<0.001) and had longer operation times (413 vs. 364 min, P<0.001) but were less likely to develop grade C POPF (0.2% vs. 1.2%, P<0.001), and had shorter length of stay (9.7 vs. 10.9 days, P<0.001). No difference in overall morbidity or peri-operative mortality was observed (72). Youngwirth et al. (73) (n=18,243) reached similar conclusions. In contrast, Aziz et al. (74) (n=1,445) found that NAT patients were more likely to have unplanned readmissions (18.0% vs. 12.2%, P=0.02) and return to theatre (2.1% vs. 1.1%, P=0.03), but no difference in peri-operative mortality was observed. The authors acknowledge that these differences may be due to more advanced disease in the NAT group (74). Teng et al. (75) (n=5,025) found that NAT was associated with longer operation times, increased transfusion requirement and higher rates of vascular reconstruction and SSI (all P<0.05). However, peri-operative mortality and major morbidity were not affected by NAT.
A recent MA by Kamarajah et al. (76) (n=19,416, 19 studies) found NAT was associated with reduced rates of overall POPF (OR =0.57; P<0.001) and grade B/C POPF (OR =0.55; P<0.001). Mangieri et al. (n=10,665) (77) and Marchegiani et al. (78) (n=455) reached the same conclusion. The latter also found that NAT was associated with reduced risk of PPH (9.1% vs. 14.6%, P=0.02) but increased risk of DGE (11.5% vs. 2.9%, P=0.03).
In summary, the use of NAT in the management of PDAC remains controversial. Evidence if emerging which suggests NAT offers a survival benefit and may be useful for identifying appropriate PD candidates. There is also evidence which suggests NAT is associated with reduced length of stay, as well as overall morbidity, POPF and PPH rates. NAT may increase DGE rates and is associated with increased rates of venous resection although this likely reflects pre-operative disease stage. Whether NAT affects unplanned readmission rate remains controversial.
Biliary stenting
This topic is well studied but remains controversial. UK national guidelines advise against routine pre-operative biliary stenting (PBS) prior to PD as the associated risks are thought to outweigh the potential benefits (63). Gong et al. (79) recently carried out a MA (27 studies, n=10,445) and found PBS was associated with increased overall morbidity (OR =1.22; P=0.01), DGE (OR =1.21; P=0.02) and SSI (OR =2.06; P<0.0001), but there was no difference in overall mortality or major morbidity. The authors concluded that patients awaiting PD should not undergo PBS unless they have cholangitis or organ failure secondary to an obstructed biliary system (79). In those who did undergo PBS, there was no difference in morbidity between those who underwent endoscopic drainage and those who underwent percutaneous drainage (79). In another recent MA, Scheufele et al. (80) (25 studies, n=6,214) found that PBS was associated with increased overall morbidity (OR =1.4; P<0.002).
Numerous single/multi-centre studies have investigated the impact of PBS on PD outcomes. Morris-Stiff et al. (81) (n=280) found stenting did not significantly alter pre-operative serum bilirubin, and that stented patients had higher overall morbidity (54% vs. 41%, P=0.03), and rates of POPF (26% vs. 18%, P=0.03) and intra-abdominal haemorrhage (12.7% vs. 5.6%, P=0.03). Hamidi et al. (82), who excluded NAT patients, matched 927 PD patients with obstructive jaundice who underwent PBS to 927 who did not. No significant difference in short-term outcomes was observed between the two groups. The authors concluded that PBS is safe in those with obstructive jaundice and that it does not need to be avoided (82). De Pastena et al. (n=1,500) found that major morbidity and mortality rates were not affected by PBS but did argue that jaundiced patients with a serum bilirubin >7.5 mg/dL should be considered for PBS.
El Nakeeb et al. (83) (n=588) found that PBS was associated with higher overall morbidity (32.5% vs. 24.1%, P=0.03), and higher risk of POPF (18.8% vs. 9.8%, P=0.002) and bile leak (10.5% vs. 5.8%, P=0.04). Mean length of stay was also longer in the drainage group (10 vs. 8 days, P=0.01). Sahora et al. (84) (n=1,000) showed that SSI rates were higher in stented patients (19% vs. 9%, P=0.001) but PBS did not affect overall morbidity or mortality. In contrast, Bolm et al. (85) matched 480 patients who underwent PBS to 480 who underwent upfront surgery (jaundiced and non-jaundiced patients were included) and found PBS was associated with increased major morbidity (27% vs. 22%, P=0.027). However, this was not significant in PBS patients who presented with jaundice. Gavazzi et al. (86) (n=180) found PBS was associated with deep SSI (13.6% vs. 4.4%, P=0.038) but not superficial SSI. Bhatti et al. (87) (n=133) found that patients undergoing PBS were more likely to develop SSI (22.7% vs. 7.4%, P=0.01) or be re-admitted (10.6% vs. 0%, P=0.006), but that PBS did not affect rates of overall peri-operative mortality or grade B/C POPF.
In summary, PBS appears to be associated with higher rates of overall morbidity, DGE, SSI, POPF, bile leak and unplanned readmission. Stented patients may also have longer length of stay. Most authors argue that patients should only undergo PBS if there is a clear indication e.g., cholangitis or organ failure secondary to jaundice. It is important to consider that patients who undergo PBS may be in a worse pre-morbid state than those who undergo upfront surgery and these patients may have higher morbidity rates regardless of their management. It is unknown whether PBS affects long-term PD outcomes.
Pre-operative blood tests
Bilirubin
Multiple prior studies have investigated the impact of serum bilirubin levels on PD outcomes. Scheufele et al. (88) (n=304) found that pre-operative bilirubin did not affect overall morbidity or long-term survival. Pamecha et al. (89) (n=177) reached similar conclusions but found severe jaundice (≥15 mg/dL) was associated with increased intra-operative blood loss (650 vs. 300 mL, P<0.001). Wang et al. (90) also reached similar conclusions but found severe jaundice was associated with increased infective complications (56.6% vs. 36.06%, P<0.05). Dolejs et al. (91) (n=2,556) found that pre-operative bilirubin level did not affect overall morbidity, major morbidity, or peri-operative mortality. Yoon et al. (92) (n=164) found that pre-operative bilirubin was more likely to be ≥7 mg/dL in those who did not survive 60-months (43.5% vs. 5.3%, P=0.01). In summary, whether pre-operative serum bilirubin affects short- and/or long-term PD outcomes remains controversial.
CRP
Pre-operative CRP levels are inversely proportional to survival in a number of cancers. Stevens et al. (93) carried out a SR to investigate the role of pre-operative CRP as a prognostic predictor in PDAC patients (n=485). Of the 6 studies which investigated the effect of high CRP on OS, whilst the cut-off value for high CRP varied, 4 suggested a correlation between high CRP and decreased OS. On multivariate analysis, 3 studies observed this finding. The authors concluded that there was insufficient evidence to justify the use of CRP level in clinical decision making. A more recent study by Mansukhani et al. (94) (n=133), where CRP levels were taken 48-h prior to surgery, found that CRP was a predictor of infective complications (P<0.01). However, this did not remain significant following multivariate analysis. In summary, high pre-operative CRP may correlate with reduced OS but this does not appear to significantly affect peri-operative outcomes.
Albumin
Serum albumin level is often used as a crude indicator of nutritional status and hepatic synthetic function. Low levels are associated with poor surgical outcomes (95). Rungsakulkij et al. (95) (n=238) found low pre-operative serum albumin was a risk factor for major morbidity (OR =0.943; P<0.05). Other studies have also found this (96,97). Hendifar et al. (98) (n=106) found low serum albumin was associated with increased post-operative transfusion rate (P=0.021) and reduced OS (HR =0.48; P=0.023). In summary, few recent studies have investigated the impact of low pre-operative serum albumin on PD outcomes but it would appear this is associated with worse short- and long-term outcomes.
CAR
CAR has been used as a marker for chronic inflammation and nutritional status. Few recent studies have investigated the impact of pre-operative CAR on PD outcomes. van Wijk et al. (99) (n=163, HR =1.745; P=0.004) and Haruki et al. (100) (n=113, P=0.049), found that, independent of staging, high CAR was a risk factor for reduced OS. No recent studies have investigated the impact of pre-operative CAR on peri-operative outcomes.
NLR
High pre-operative NLR is associated with poor prognosis in cancer patients across a wide spectrum of diagnoses, stages of disease, and courses of treatment (101). Although this is well described, the mechanisms behind this are poorly understood. Following a recent MA, Mowbray et al. (102) (8 studies, n=1,519) found high pre-operative NLR was associated with reduced OS (HR =1.77; P<0.001). The authors concluded that further studies are required to obtain a cut-off value which can be used for prognostic purposes (102). Sun et al. (103) (n=358) found OS was lower in patients with NLR >3.32 (HR =1.6; P=0.013).
Concerning peri-operative outcomes, Arikan et al. (104) (n=123) demonstrated that high pre-operative NLR was associated with increased overall morbidity following PD (41.9% vs. 14.8%, P=0.032). NLR had a high specificity but low sensitivity for predicting POPF (104). Other authors have also found this (105). In addition, Ida et al. (106) (n=208) found high NLR was associated with increased overall morbidity (OR =1.13; P=0.03) which contributed towards increased length of stay in those who experienced a complication (19 vs. 33 days, P=0.005). Huang et al. (107) (n=223) also concluded that patients who experienced complications were more likely to have a NLR ≥3.78 (3.38 vs. 2.24, P=0.006). Shen et al. (108) (n=835) found that NLR was significantly higher in those who experienced major morbidity (3.75 vs. 2.98, P<0.001). In summary, high NLR appears to increase peri-operative morbidity and reduce OS but a clinically significant threshold is yet to be defined.
Discussion
This review was carried out to consolidate the recent literature on pre-selected pre-operative factors and their impact on PD outcomes. Table 1 summaries the impact of each variable on selected outcomes. An appreciation for the modifiable factors discussed may allow for patient optimisation prior to surgery. For example, a pre-operative review of all patients with diabetes or COPD by a specialist nurse, or the use of CPET to plan for peri-operative care, may result in reductions to morbidity rates. This, in turn, may increase the likelihood of patients starting and/or completing adjuvant chemotherapy. Routine assessment of pre-operative CT imaging for sarcopenia and/or myosteatosis could prompt early dietetic input to reduce the pre-operative catabolic state, which may reduce the risk of anastomotic failure. To our knowledge, no prior studies have investigated the impact of treating myosteatosis on PD outcomes. We argue a study is required where patients with myosteatosis are randomised to either a specialised diet and exercise programme or standard care prior to surgery to investigate the impact on morbidity.
Table 1
| Pre-operative factor | Risk of POPF | Risk of SSI | Risk of DGE | Intra-operative blood loss | Length of stay | Peri-operative morbidity | Peri-operative mortality | Disease-free survival | OS |
|---|---|---|---|---|---|---|---|---|---|
| Demographic factors | |||||||||
| Advanced age (various thresholds) | ↑ | ↑ | |||||||
| Male gender | ↑ | ↑ | |||||||
| Pre-existing comorbidities | |||||||||
| Cardiac | ↑ | ↑ | |||||||
| Respiratory | ↑ | ↑ | |||||||
| DM | |||||||||
| Nutritional status | |||||||||
| BMI ≤18.5 kg/m2 | ↑ | ↑ | ↑ | ||||||
| BMI ≥25 kg/m2 | ↑ | ↑ | ↑ | ↑ | |||||
| BMI ≥30 kg/m2 | ↑ | ↑ | ↑ | ↑ | |||||
| BMI ≥40 kg/m2 | ↑ | ||||||||
| Sarcopenia | ↑ | ↑ | ↑ | ↑ | ↓ | ||||
| Myosteatosis | ↓ | ||||||||
| Pre-operative imaging | |||||||||
| Heterogeneous tumour on CT | ↓ | ||||||||
| Hypoattenuating tumour on CT | ↓ | ||||||||
| Low enhancement change on dynamic contrast-enhanced CT | ↓ | ↓ | |||||||
| Rim-enhancement on MRI | ↓ | ↓ | |||||||
| Max. standardised uptake value >3.5 on PET-CT | ↓ | ↓ | |||||||
| Metabolic tumour volume >3 cm3 on PET-CT | ↓ | ↓ | |||||||
| Total lesion glycolysis >10 g on PET-CT | ↓ | ↓ | |||||||
| Pre-operative therapies | |||||||||
| Biliary stenting | ↑ | ↑ | ↑ | ↑ | |||||
| NAT | ↓ | ↑ | ↓ | ↓ | ↑ | ||||
| Pre-operative blood tests | |||||||||
| Bilirubin <7 mg/dL | ↑ | ||||||||
| Bilirubin >20 mg/dL | ↑ | ||||||||
| Raised CRP (various thresholds) | ↓ | ||||||||
| Albumin <35 g/L | ↑ | ↑ | ↓ | ↓ | |||||
| Raised CAR (various thresholds) | ↑ | ↑ | ↓ | ↓ | |||||
| Raised NLR (various thresholds) | ↑ | ↑ | ↑ | ↓ |
Increased or decreased risk/survival compared to patients without the factor. References can be found within the article text. PD, pancreaticoduodenectomy; POPF, postoperative pancreatic fistula; SSI, surgical site infection; DGE, delayed gastric emptying; OS, overall survival; DM, diabetes mellitus; BMI, body mass index; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; NAT, neoadjuvant therapy; CRP, C-reactive protein; CAR, C-reactive protein/albumin ratio; NLR, neutrophil/lymphocyte ratio.
An appreciation for the non-modifiable factors discussed will assist the assessment of potential surgical candidates, allow clinicians to consider the appropriateness of PD, and result in more informed risk stratification and discussions with patients regarding risk and benefit. The influence of many of these factors on outcomes are limited to single-centre retrospective analyses and may not account for all confounding variables. The factors discussed were selected as they are currently being investigated by the RAW study. This is an international, multi-centre, retrospective analysis, which aims to investigate the impact of the variables discussed on patterns of recurrence and surgical outcomes following PD (NCT04596865). Results are expected in 2022.
This review has not aimed to answer a specific research question. Rather, it aims to provide the reader with a broad overview. Due to the number of topics covered, certain sections are very concise. Furthermore, we have chosen the variables which will be investigated by the RAW study and acknowledge that there are other important variables which affect PD outcomes e.g., smoking status. For simplicity, we have not included studies with less than 100 cases and limited our search to English language articles on the PubMed database. We acknowledge that out search methods will have been influenced by selection and publication bias and that there is a high degree of heterogeneity between the included studies which has not been formally addressed. Furthermore, sensitivity analysis, reporting bias and certainty assessments were not carried out. Meta-analysis has not been performed due to the large number of topics and studies.
Conclusions
Despite improvements to patient selection, surgical technique, and peri-operative care, PD continues to be associated with considerable morbidity. Even in the absence of surgical complications, few patients achieve long-term survival due to disease recurrence. A number of the variables discussed affect PD outcomes. Some of these may be used as prognostic indicators to assist patient selection, optimise patients pre-operatively and to guide risk/benefit discussions with potential surgical candidates. A robust study, which considers confounding variables, is required to further investigate these.
Acknowledgments
The authors would like to thank Dr. Mark Puckett (Consultant Radiologist, University Hospitals Plymouth NHS Trust, Plymouth, UK) for advising on the radiology section.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apc-21-15
Peer Review File: Available at https://dx.doi.org/10.21037/apc-21-15
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apc-21-15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This review was not registered and a protocol was not prepared.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019;10:10-27. [Crossref] [PubMed]
- Levi ST, Gough BL, Darcy CE, et al. Pancreatic resections: 30 and 90-day outcomes in octogenarians. Surg Oncol 2021;37:101319 [Crossref] [PubMed]
- Shamali A, De'Ath HD, Jaber B, et al. Elderly patients have similar short term outcomes and five-year survival compared to younger patients after pancreaticoduodenectomy. Int J Surg 2017;45:138-43. [Crossref] [PubMed]
- El Nakeeb A, Atef E, El Hanafy E, et al. Outcomes of pancreaticoduodenectomy in elderly patients. Hepatobiliary Pancreat Dis Int 2016;15:419-27. [Crossref] [PubMed]
- Zhang D, Gao J, Li S, et al. Outcome after pancreaticoduodenectomy for malignancy in elderly patients. Hepatogastroenterology 2015;62:451-4. [PubMed]
- Wiltberger G, Muhl B, Benzing C, et al. Preoperative risk stratification for major complications following pancreaticoduodenectomy: Identification of high-risk patients. Int J Surg 2016;31:33-9. [Crossref] [PubMed]
- Gruppo M, Tolin F, Franzato B, et al. Impact of Age on Short- and Long-Term Outcomes after Pancreatoduodenectomy for Periampullary Neoplasms. Gastroenterol Res Pract 2020;2020:1793051 [Crossref] [PubMed]
- Miyazaki Y, Kokudo T, Amikura K, et al. Age does not affect complications and overall survival rate after pancreaticoduodenectomy: Single-center experience and systematic review of literature. Biosci Trends 2016;10:300-6. [Crossref] [PubMed]
- Renz BW, Khalil PN, Mikhailov M, et al. Pancreaticoduodenectomy for adenocarcinoma of the pancreatic head is justified in elderly patients: A Retrospective Cohort Study. Int J Surg 2016;28:118-25. [Crossref] [PubMed]
- Watanabe J, Hanaki T, Arai Y, et al. Perioperative Outcomes after Pancreaticoduodenectomy in Elderly Patients. Hepatogastroenterology 2015;62:590-4. [PubMed]
- Beltrame V, Gruppo M, Pastorelli D, et al. Outcome of pancreaticoduodenectomy in octogenarians: Single institution's experience and review of the literature. J Visc Surg 2015;152:279-84. [Crossref] [PubMed]
- Oguro S, Shimada K, Kishi Y, et al. Perioperative and long-term outcomes after pancreaticoduodenectomy in elderly patients 80 years of age and older. Langenbecks Arch Surg 2013;398:531-8. [Crossref] [PubMed]
- Kim SY, Fink MA, Perini M, et al. Age 80 years and over is not associated with increased morbidity and mortality following pancreaticoduodenectomy. ANZ J Surg 2018;88:E445-50. [Crossref] [PubMed]
- Kim SY, Weinberg L, Christophi C, et al. The outcomes of pancreaticoduodenectomy in patients aged 80 or older: a systematic review and meta-analysis. HPB (Oxford) 2017;19:475-82. [Crossref] [PubMed]
- Haigh PI, Bilimoria KY, DiFronzo LA. Early postoperative outcomes after pancreaticoduodenectomy in the elderly. Arch Surg 2011;146:715-23. [Crossref] [PubMed]
- Futagawa Y, Kanehira M, Furukawa K, et al. Study on the Validity of Pancreaticoduodenectomy in the Elderly. Anticancer Res 2017;37:5309-16. [PubMed]
- Lee DY, Schwartz JA, Wexelman B, et al. Outcomes of pancreaticoduodenectomy for pancreatic malignancy in octogenarians: an American College of Surgeons National Surgical Quality Improvement Program analysis. Am J Surg 2014;207:540-8. [Crossref] [PubMed]
- Williamsson C, Rystedt J, Andersson B. An analysis of gender differences in treatment and outcome of periampullary tumours in Sweden - A national cohort study. HPB (Oxford) 2021;23:847-53. [Crossref] [PubMed]
- Mazmudar A, Vitello D, Chapman M, et al. Gender as a risk factor for adverse intraoperative and postoperative outcomes of elective pancreatectomy. J Surg Oncol 2017;115:131-6. [Crossref] [PubMed]
- Chen YT, Deng Q, Che X, et al. Impact of body mass index on complications following pancreatectomy: Ten-year experience at National Cancer Center in China. World J Gastroenterol 2015;21:7218-24. [Crossref] [PubMed]
- Aoki S, Miyata H, Konno H, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci 2017;24:243-51. [Crossref] [PubMed]
- Tang T, Tan Y, Xiao B, et al. Influence of Body Mass Index on Perioperative Outcomes Following Pancreaticoduodenectomy. J Laparoendosc Adv Surg Tech A 2021;31:999-1005. [Crossref] [PubMed]
- El Nakeeb A, Hamed H, Shehta A, et al. Impact of obesity on surgical outcomes post-pancreaticoduodenectomy: a case-control study. Int J Surg 2014;12:488-93. [Crossref] [PubMed]
- Del Chiaro M, Rangelova E, Ansorge C, et al. Impact of body mass index for patients undergoing pancreaticoduodenectomy. World J Gastrointest Pathophysiol 2013;4:37-42. [Crossref] [PubMed]
- Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol 2011;18:2126-35. [Crossref] [PubMed]
- You L, Zhao W, Hong X, et al. The Effect of Body Mass Index on Surgical Outcomes in Patients Undergoing Pancreatic Resection: A Systematic Review and Meta-Analysis. Pancreas 2016;45:796-805. [Crossref] [PubMed]
- Ekström E, Ansari D, Williamsson C, et al. Impact of body constitution on complications following pancreaticoduodenectomy: A retrospective cohort study. Int J Surg 2017;48:116-21. [Crossref] [PubMed]
- Chang EH, Sugiyama G, Smith MC, et al. Obesity and surgical complications of pancreaticoduodenectomy: An observation study utilizing ACS NSQIP. Am J Surg 2020;220:135-9. [Crossref] [PubMed]
- Zorbas KA, Karachristos A. Impact of class III obesity on 30 days mortality and morbidity after Whipple procedure. HPB 2017;19:S156. [Crossref]
- Pausch T, Hartwig W, Hinz U, et al. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery 2012;152:S81-8. [Crossref] [PubMed]
- Tsai S, Choti MA, Assumpcao L, et al. Impact of obesity on perioperative outcomes and survival following pancreaticoduodenectomy for pancreatic cancer: a large single-institution study. J Gastrointest Surg 2010;14:1143-50. [Crossref] [PubMed]
- Dandona M, Linehan D, Hawkins W, et al. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas 2011;40:931-7. [Crossref] [PubMed]
- Gaujoux S, Torres J, Olson S, et al. Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol 2012;19:2908-16. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636-46. [Crossref] [PubMed]
- Namm JP, Thakrar KH, Wang CH, et al. A semi-automated assessment of sarcopenia using psoas area and density predicts outcomes after pancreaticoduodenectomy for pancreatic malignancy. J Gastrointest Oncol 2017;8:936-44. [Crossref] [PubMed]
- Linder N, Schaudinn A, Langenhan K, et al. Power of computed-tomography-defined sarcopenia for prediction of morbidity after pancreaticoduodenectomy. BMC Med Imaging 2019;19:32. [Crossref] [PubMed]
- Pecorelli N, Carrara G, De Cobelli F, et al. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg 2016;103:434-42. [Crossref] [PubMed]
- Jang M, Park HW, Huh J, et al. Predictive value of sarcopenia and visceral obesity for postoperative pancreatic fistula after pancreaticoduodenectomy analyzed on clinically acquired CT and MRI. Eur Radiol 2019;29:2417-25. [Crossref] [PubMed]
- Nishida Y, Kato Y, Kudo M, et al. Preoperative Sarcopenia Strongly Influences the Risk of Postoperative Pancreatic Fistula Formation After Pancreaticoduodenectomy. J Gastrointest Surg 2016;20:1586-94. [Crossref] [PubMed]
- Ryu Y, Shin SH, Kim JH, et al. The effects of sarcopenia and sarcopenic obesity after pancreaticoduodenectomy in patients with pancreatic head cancer. HPB (Oxford) 2020;22:1782-92. [Crossref] [PubMed]
- Takagi K, Yoshida R, Yagi T, et al. Radiographic sarcopenia predicts postoperative infectious complications in patients undergoing pancreaticoduodenectomy. BMC Surg 2017;17:64. [Crossref] [PubMed]
- Stretch C, Aubin JM, Mickiewicz B, et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS One 2018;13:e0196235 [Crossref] [PubMed]
- Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478-86. [Crossref] [PubMed]
- Gruber ES, Jomrich G, Tamandl D, et al. Sarcopenia and sarcopenic obesity are independent adverse prognostic factors in resectable pancreatic ductal adenocarcinoma. PLoS One 2019;14:e0215915 [Crossref] [PubMed]
- Murnane LC, Forsyth AK, Koukounaras J, et al. Myosteatosis predicts higher complications and reduced overall survival following radical oesophageal and gastric cancer surgery. Eur J Surg Oncol 2021;47:2295-303. [Crossref] [PubMed]
- West MA, van Dijk DPJ, Gleadowe F, et al. Myosteatosis is associated with poor physical fitness in patients undergoing hepatopancreatobiliary surgery. J Cachexia Sarcopenia Muscle 2019;10:860-71. [Crossref] [PubMed]
- Lv X, Qiao W, Leng Y, et al. Impact of diabetes mellitus on clinical outcomes of pancreatic cancer after surgical resection: A systematic review and meta-analysis. PLoS One 2017;12:e0171370 [Crossref] [PubMed]
- Nakata B, Ishikawa T, Amano R, et al. Impact of preoperative diabetes mellitus on clinical outcome after pancreatectomy. Int J Surg 2013;11:757-61. [Crossref] [PubMed]
- Xia X, Huang C, Cen G, et al. Preoperative diabetes as a protective factor for pancreatic fistula after pancreaticoduodenectomy: a meta-analysis. Hepatobiliary Pancreat Dis Int 2015;14:132-8. [Crossref] [PubMed]
- King JT Jr, Goulet JL, Perkal MF, et al. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg 2011;253:158-65. [Crossref] [PubMed]
- Berbudi A, Rahmadika N, Tjahjadi AI, et al. Type 2 Diabetes and its Impact on the Immune System. Curr Diabetes Rev 2020;16:442-9. [Crossref] [PubMed]
- Ronnekleiv-Kelly SM, Greenblatt DY, Lin CP, et al. Impact of cardiac comorbidity on early outcomes after pancreatic resection. J Gastrointest Surg 2014;18:512-22. [Crossref] [PubMed]
- de la Fuente SG, Bennett KM, Pappas TN, et al. Pre- and intraoperative variables affecting early outcomes in elderly patients undergoing pancreaticoduodenectomy. HPB (Oxford) 2011;13:887-92. [Crossref] [PubMed]
- DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: A novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg 2006;244:931-7; discussion 937-9. [Crossref] [PubMed]
- Shia BC, Qin L, Lin KC, et al. Age comorbidity scores as risk factors for 90-day mortality in patients with a pancreatic head adenocarcinoma receiving a pancreaticoduodenectomy: A National Population-Based Study. Cancer Med 2020;9:562-74. [Crossref] [PubMed]
- Lee S, Kim SH, Park HK, et al. Pancreatic Ductal Adenocarcinoma: Rim Enhancement at MR Imaging Predicts Prognosis after Curative Resection. Radiology 2018;288:456-66. [Crossref] [PubMed]
- Kim HS, Kim YJ, Kim KG, et al. Preoperative CT texture features predict prognosis after curative resection in pancreatic cancer. Sci Rep 2019;9:17389. [Crossref] [PubMed]
- Zhu L, Shi X, Xue H, et al. CT Imaging Biomarkers Predict Clinical Outcomes After Pancreatic Cancer Surgery. Medicine (Baltimore) 2016;95:e2664 [Crossref] [PubMed]
- Cassinotto C, Chong J, Zogopoulos G, et al. Resectable pancreatic adenocarcinoma: Role of CT quantitative imaging biomarkers for predicting pathology and patient outcomes. Eur J Radiol 2017;90:152-8. [Crossref] [PubMed]
- Choi HJ, Kang CM, Lee WJ, et al. Prognostic value of 18F-fluorodeoxyglucose positron emission tomography in patients with resectable pancreatic cancer. Yonsei Med J 2013;54:1377-83. [Crossref] [PubMed]
- Yamamoto T, Sugiura T, Mizuno T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol 2015;22:677-84. [Crossref] [PubMed]
- Lee JW, Kang CM, Choi HJ, et al. Prognostic Value of Metabolic Tumor Volume and Total Lesion Glycolysis on Preoperative 18F-FDG PET/CT in Patients with Pancreatic Cancer. J Nucl Med 2014;55:898-904. [Crossref] [PubMed]
- O'Reilly D, Fou L, Hasler E, et al. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018;18:962-70. [Crossref] [PubMed]
- Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol 2019;49:190-4. [Crossref] [PubMed]
- Ghaneh P, Palmer DH, Cicconi S, et al. ESPAC-5F: Four-arm, prospective, multicenter, international randomized phase II trial of immediate surgery compared with neoadjuvant gemcitabine plus capecitabine (GEMCAP) or FOLFIRINOX or chemoradiotherapy (CRT) in patients with borderline resectable pancreatic cancer. J Clin Oncol 2020;38:4505. [Crossref]
- Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol 2020;38:1763-73. [Crossref] [PubMed]
- Rangarajan K, Pucher PH, Armstrong T, et al. Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: a systematic review and meta-analysis. Ann R Coll Surg Engl 2019;101:453-62. [Crossref] [PubMed]
- Greco SH, August DA, Shah MM, et al. Neoadjuvant therapy is associated with lower margin positivity rates after Pancreaticoduodenectomy in T1 and T2 pancreatic head cancers: An analysis of the National Cancer Database. Surg Open Sci 2020;3:22-8. [Crossref] [PubMed]
- Lee YS, Lee JC, Yang SY, et al. Neoadjuvant therapy versus upfront surgery in resectable pancreatic cancer according to intention-to-treat and per-protocol analysis: A systematic review and meta-analysis. Sci Rep 2019;9:15662. [Crossref] [PubMed]
- Kamarajah SK, Naffouje SA, Salti GI, et al. Neoadjuvant Chemotherapy for Pancreatic Ductal Adenocarcinoma is Associated with Lower Post-Pancreatectomy Readmission Rates: A Population-Based Cohort Study. Ann Surg Oncol 2021;28:1896-905. [Crossref] [PubMed]
- Cho SW, Tzeng CW, Johnston WC, et al. Neoadjuvant radiation therapy and its impact on complications after pancreaticoduodenectomy for pancreatic cancer: analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). HPB (Oxford) 2014;16:350-6. [Crossref] [PubMed]
- Cools KS, Sanoff HK, Kim HJ, et al. Impact of neoadjuvant therapy on postoperative outcomes after pancreaticoduodenectomy. J Surg Oncol 2018;118:455-62. [Crossref] [PubMed]
- Youngwirth LM, Nussbaum DP, Thomas S, et al. Nationwide trends and outcomes associated with neoadjuvant therapy in pancreatic cancer: An analysis of 18 243 patients. J Surg Oncol 2017;116:127-32. [Crossref] [PubMed]
- Aziz H, Zeeshan M, Jie T, et al. Neoadjuvant Chemoradiation Therapy Is Associated with Adverse Outcomes in Patients Undergoing Pancreaticoduodenectomy for Pancreatic Cancer. Am Surg 2019;85:1276-80. [Crossref] [PubMed]
- Teng A, Lee DY, Yang CK, et al. The effects of neoadjuvant chemoradiation on pancreaticoduodenectomy—the American College of Surgeon's National Surgical Quality Improvement Program analysis. J Surg Res 2015;196:67-73. [Crossref] [PubMed]
- Kamarajah SK, Bundred JR, Boyle C, et al. Impact of neoadjuvant therapy on post-operative pancreatic fistula: a systematic review and meta-analysis. ANZ J Surg 2020;90:2201-10. [Crossref] [PubMed]
- Mangieri CW, Strode MA, Moaven O, et al. Utilization of chemoradiation therapy provides strongest protective effect for avoidance of postoperative pancreatic fistula following pancreaticoduodenectomy: A NSQIP analysis. J Surg Oncol 2020;122:1604-11. [Crossref] [PubMed]
- Marchegiani G, Andrianello S, Nessi C, et al. Neoadjuvant Therapy Versus Upfront Resection for Pancreatic Cancer: The Actual Spectrum and Clinical Burden of Postoperative Complications. Ann Surg Oncol 2018;25:626-37. [Crossref] [PubMed]
- Gong L, Huang X, Wang L, et al. The effect of preoperative biliary stents on outcomes after pancreaticoduodenectomy: A meta-analysis. Medicine (Baltimore) 2020;99:e22714 [Crossref] [PubMed]
- Scheufele F, Schorn S, Demir IE, et al. Preoperative biliary stenting versus operation first in jaundiced patients due to malignant lesions in the pancreatic head: A meta-analysis of current literature. Surgery 2017;161:939-50. [Crossref] [PubMed]
- Morris-Stiff G, Tamijmarane A, Tan YM, et al. Pre-operative stenting is associated with a higher prevalence of post-operative complications following pancreatoduodenectomy. Int J Surg 2011;9:145-9. [Crossref] [PubMed]
- Hamidi M, Dauch J, Watson R, et al. Outcomes with Preoperative Biliary Stenting After Pancreaticoduodenectomy In the Modern Era. J Gastrointest Surg 2021;25:162-8. [Crossref] [PubMed]
- El Nakeeb A, Salem A, Mahdy Y, et al. Value of preoperative biliary drainage on postoperative outcome after pancreaticoduodenectomy: A case-control study. Asian J Surg 2018;41:155-62. [Crossref] [PubMed]
- Sahora K, Morales-Oyarvide V, Ferrone C, et al. Preoperative biliary drainage does not increase major complications in pancreaticoduodenectomy: a large single center experience from the Massachusetts General Hospital. J Hepatobiliary Pancreat Sci 2016;23:181-7. [Crossref] [PubMed]
- Bolm L, Petrova E, Woehrmann L, et al. The impact of preoperative biliary stenting in pancreatic cancer: A case-matched study from the German nationwide pancreatic surgery registry (DGAV StuDoQ|Pancreas). Pancreatology 2019;19:985-93. [Crossref] [PubMed]
- Gavazzi F, Ridolfi C, Capretti G, et al. Role of preoperative biliary stents, bile contamination and antibiotic prophylaxis in surgical site infections after pancreaticoduodenectomy. BMC Gastroenterol 2016;16:43. [Crossref] [PubMed]
- Bhatti ABH, Jafri RZ, Khan MK, et al. Preoperative Endoscopic Biliary Stenting Before Pancreaticoduodenectomy: Does Timing Matter? Surg Innov 2021;28:567-72. [Crossref] [PubMed]
- Scheufele F, Aichinger L, Jäger C, et al. INR and not bilirubin levels predict postoperative morbidity in patients with malignant obstructive jaundice. Am J Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Pamecha V, Sadashiv Patil N, Kumar S, et al. Upfront pancreaticoduodenectomy in severely jaundiced patients: is it safe? J Hepatobiliary Pancreat Sci 2019;26:524-33. [Crossref] [PubMed]
- Wang S, Wang X, Li L, et al. Association of preoperative obstructive jaundice with postoperative infectious complications following pancreaticoduodenectomy. Hepatogastroenterology 2013;60:1274-9. [PubMed]
- Dolejs S, Zarzaur BL, Zyromski NJ, et al. Does Hyperbilirubinemia Contribute to Adverse Patient Outcomes Following Pancreatoduodenectomy? J Gastrointest Surg 2017;21:647-56. [Crossref] [PubMed]
- Yoon KW, Heo JS, Choi DW, et al. Factors affecting long-term survival after surgical resection of pancreatic ductal adenocarcinoma. J Korean Surg Soc 2011;81:394-401. [Crossref] [PubMed]
- Stevens L, Pathak S, Nunes QM, et al. Prognostic significance of pre-operative C-reactive protein and the neutrophil-lymphocyte ratio in resectable pancreatic cancer: a systematic review. HPB (Oxford) 2015;17:285-91. [Crossref] [PubMed]
- Mansukhani V, Desai G, Shah R, et al. The role of preoperative C-reactive protein and procalcitonin as predictors of post-pancreaticoduodenectomy infective complications: A prospective observational study. Indian J Gastroenterol 2017;36:289-95. [Crossref] [PubMed]
- Rungsakulkij N, Tangtawee P, Suragul W, et al. Correlation of serum albumin and prognostic nutritional index with outcomes following pancreaticoduodenectomy. World J Clin Cases 2019;7:28-38. [Crossref] [PubMed]
- Yamamoto M, Kawaguchi Y, Ichida A, et al. Evaluation of preoperative nutritional variables to predict postoperative complications after pancreaticoduodenectomy. Nutrition 2019;67-68S:100006.
- Xu W, Peng X, Jiang B. Hypoalbuminemia after pancreaticoduodenectomy does not predict or affect short-term postoperative prognosis. BMC Surg 2020;20:72. [Crossref] [PubMed]
- Hendifar A, Osipov A, Khanuja J, et al. Influence of Body Mass Index and Albumin on Perioperative Morbidity and Clinical Outcomes in Resected Pancreatic Adenocarcinoma. PLoS One 2016;11:e0152172 [Crossref] [PubMed]
- van Wijk L, de Klein GW, Kanters MA, et al. The ultimate preoperative C-reactive protein-to-albumin ratio is a prognostic factor for survival after pancreatic cancer resection. Eur J Med Res 2020;25:46. [Crossref] [PubMed]
- Haruki K, Shiba H, Shirai Y, et al. The C-reactive Protein to Albumin Ratio Predicts Long-Term Outcomes in Patients with Pancreatic Cancer After Pancreatic Resection. World J Surg 2016;40:2254-60. [Crossref] [PubMed]
- Cupp MA, Cariolou M, Tzoulaki I, et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med 2020;18:360. [Crossref] [PubMed]
- Mowbray NG, Griffith D, Hammoda M, et al. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB (Oxford) 2018;20:379-84. [Crossref] [PubMed]
- Sun S, He C, Wang J, et al. The prognostic significance of inflammation-based scores in patients with ampullary carcinoma after pancreaticoduodenectomy. BMC Cancer 2020;20:981. [Crossref] [PubMed]
- Arikan TB, Sözüer EM, Topal U, et al. The value and prognostic significance of neutrophil / lymphocyte ratio in predicting pancreatic fistula in patients undergoing pancreaticoduodenectomy for periampullary tumors. Ann Med Res 2021;27:1214-20. [Crossref]
- Fang LP, Xu XY, Ji Y, et al. The Prognostic Value of Preoperative Neutrophil-to-Lymphocyte Ratio in Resected Patients with Pancreatic Adenocarcinoma. World J Surg 2018;42:3736-45. [Crossref] [PubMed]
- Ida M, Tachiiri Y, Sato M, et al. Neutrophil-to-lymphocyte ratio as indicator to severe complication after pancreaticoduodenectomy or distal pancreatectomy. Acta Anaesthesiol Scand 2019;63:739-44. [Crossref] [PubMed]
- Huang H, Wang C, Ji F, et al. Nomogram based on albumin and neutrophil-to-lymphocyte ratio for predicting postoperative complications after pancreaticoduodenectomy. Gland Surg 2021;10:877-91. [Crossref] [PubMed]
- Shen Z, Xu Z, Wang W, et al. A novel nomogram for predicting the risk of major complications after pancreaticoduodenectomy in patients with obstructive jaundice. Clin Chim Acta 2021;517:162-70. [Crossref] [PubMed]
Cite this article as: Russell TB, Labib PL, Aroori S. Selected pre-operative factors which affect pancreaticoduodenectomy outcomes: a systematic review. Ann Pancreat Cancer 2021;4:10.


