
Selected intra-operative factors which affect pancreaticoduodenectomy outcomes: a narrative review
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the eleventh most common cancer worldwide and its incidence is set to rise (1). It is projected to become the UK’s fourth biggest cancer killer by 2030, with similar trends expected in high-income countries within Europe, North America and Oceania (1). Prognosis following a PDAC diagnosis is extremely poor; five-year survival is less than 10% (1). In selected patients with early disease, PDAC affecting the head of the pancreas can be treated with pancreaticoduodenectomy (PD) with curative intent. This high-risk operation is associated with considerable morbidity and up to 80% of patients develop recurrent disease. This article reviews the recent literature on selected intra-operative factors and their impact on short- and long-term PD outcomes. Although many of the factors discussed are non-modifiable, an in-depth understanding of their impact will improve patient selection and guide risk/benefit discussions. An appreciation for the modifiable factors discussed will allow surgeons to consider their operative technique and potentially optimise their outcomes. We present the following article in accordance with the Narrative Review reporting checklist (available at https://apc.amegroups.com/article/view/10.21037/apc-21-16/rc).
Methods
The factors included were selected prior to carrying out the literature search. These were: pylorus-resecting versus pylorus-resecting technique, open versus minimally invasive technique, type of pancreatic anastomosis, concomitant vascular resection, pancreas texture, evidence of pancreatitis, dilatation of the pancreatic duct, and peri-operative blood transfusion. American Society of Anesthesiologists (ASA) grade was also included although this not strictly an intra-operative factor. In the blood transfusion section, articles reporting on transfusions given in the intra- and early post-operative periods were included. Pre-operative factors have not been included as these have been covered in a separate article.
A comprehensive online search of the English literature was carried out on 14th June 2021. The PubMed database was searched using the terms “factor in question”, “pancreaticoduodenectomy”, and “outcome” from May 2011 through May 2021. The following articles were included: (I) English language; (II) human studies; (III) meta-analyses (MAs), systematic reviews and clinical studies reporting on outcomes of PD performed for suspected PDAC; (IV) in terms of risk factors/associations, only statistically significant results were included (P<0.05).
Results
ASA grade
The ASA physical classification system, or ASA grade, has been in use for more than 60 years. Its purpose is to categorise a patient’s pre-operative physiological status to guide clinical decision making. The system alone cannot quantify risk since it does not consider the operation being performed or physical factors such as a difficult airway, or a patient’s wish to refuse a blood transfusion. Furthermore, it is subjective and does not consider the impact of advancing age on physiological fitness. Nonetheless, it is a useful tool. ASA grade I patients are healthy and grade II patients have mild systemic disease (2). Grade III patients have severe systemic disease and grade IV patients have severe systemic disease which is a constant threat to life (2). It is very rare for patients with an ASA grade of IV or above to be offered PD.
The impact of ASA grade on surgical outcomes is well documented (2). Morbidity and mortality rates increase with ASA grade in both the elective and emergency setting (3). Specific to pancreatic surgery, increasing ASA grade has been shown to correlate with adverse outcome following PD. Eeson et al. (4) (n=100) found that ASA grade III was associated with increased peri-operative mortality (P=0.012). However, this was no longer significant once increasing age was adjusted for (4). The authors concluded that, whilst age >80 years should not be an absolute contraindication, extreme caution should be used when considering resection in a patient of this age if they are ASA grade III (4). Other authors have shown that ASA grade III patients have significantly increased major morbidity rates (5,6). Concerning long-term outcomes, the Eeson et al. (4) study showed that increasing ASA grade correlated with reduced overall survival (OS). Compared with ASA grade I/II, median OS was significantly shorter in ASA grade III patients (12.0 vs. 19.5 months, P=0.042).
In summary, ASA grade is a basic but useful tool for estimating risk in surgical candidates. One should consider the additional risks when offering PD to those with ASA grade ≥III, especially older patients. These patients have higher peri-operative morbidity and mortality rates, and reduced OS.
Pylorus-resecting versus pylorus-preserving technique
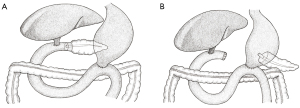
The classic PD (Figure 1A) refers to resection of the pancreatic head, gallbladder (and bile duct), duodenum, and the distal stomach, before formation of the three anastomoses. A modified approach is the pylorus-preserving PD (PPPD) which, as the name suggests, does not involve resection of the distal stomach (Figure 1B). The PPPD was popularised by the American surgeons L. William Traverso and William Longmire in the late 1970s and was initially intended for the management of chronic pancreatitis (7). Due to reports of shorter operation times, decreased intra-operative blood loss, and reduced incidence of dumping syndrome, it was proposed as an alternative to the classic approach for the management of peri-ampullary malignancies (7).

Multiple MAs have compared the outcomes of the two procedures. Whilst there were initial concerns regarding the oncological outcomes of PPPD, it has been shown to be equivalent in terms of recurrence and long-term survival (8). Following a review of eight randomised controlled trials (RCTs), Hüttner et al. (9) (n=512) found that a pylorus-preserving technique did not affect morbidity, mortality or OS. A less recent MA by Diener et al. (10) reached similar conclusions. A further MA by Yang et al. (11) (eight RCTs, n=662), suggested PPPD had short-term advantages, including reduced operation time (mean difference: 53 min; P=0.01) and reduced intra-operative blood loss (mean difference: 365 mL; P=0.006). However, classic PD was associated with a lower rate of delayed gastric emptying (DGE; RR =2.35; P=0.04). Morbidity and mortality rates were similar, and OS was not studied (11). A MA by Zhou et al. (12) reached similar conclusions.
In summary, the classic PD and the PPPD are both acceptable approaches which have similar recurrence and survival rates. It may be that the PPPD is associated with shorter operation times and reduced intra-operative blood loss. Classic PD may be associated with a reduced rate of DGE.
Open versus minimally invasive techniques
In recent decades, minimally invasive surgical techniques have seen a meteoric rise. Many operations which were once performed open are now routinely performed laparoscopically or robotically. The first laparoscopic PD (LPD) was reported by Michel Gagner and Alfons Pomp in 1994. However, uptake has been slow due to the associated technical challenges of performing an oncological resection in a difficult-to-access anatomical location, and the need to perform three anastomoses as part of the reconstruction (13). Not all PD candidates are suitable for a laparoscopic resection; this includes those who are likely to require concomitant vascular resection, are obese, or have previously had abdominal surgery or pancreatitis (14). Unsurprisingly, a high proportion (up to 10%) of laparoscopic procedures are converted to open (15). Multiple studies, including MA, have suggested that LPD is associated with longer operation times, shorter length of stay and reduced intra-operative blood loss (14,16-19). No statistically significant difference has been observed in terms of morbidity, mortality, or oncological outcomes (15,17,20). Despite this, some specialists have concerns regarding the risk of major morbidity following LPD. A recent RCT by van Hilst et al. (21) was terminated early due to safety concerns as, although not statistically significant, LPD was associated with a higher number of complication-related deaths.
Laparoscopic surgery creates several issues for the surgeon, including two-dimensional imaging, poor ergonomics, restricted range of movement, and a long learning curve. Robotic PD (RPD) has been developed as an alternative which has become popular in certain units. This method provides superior three-dimensional visualisation, instruments which mimic the surgeon’s own hands, an articulating “wrist”, and greater range of motion. The feasibility of RPD was first described in 2003 by Giulianotti et al. (22) who published a series of thirteen patients. A recent MA by Da Dong et al. (23) (24 studies, n=12,579) compared RPD to open surgery. RPD was associated with reduced intra-operative blood loss (mean difference: 191 mL; P<0.001) and longer operation time (mean difference: 75 min; P<0.001). There was a strong association toward increased complete (R0) resection rate in the RPD group but this was not quite significant (15.6% vs. 19.9%, P=0.05). The surgical approach did not impact on major morbidity or mortality rates, and long-term outcomes were not studied (23). Survival outcomes following RPD are not well studied. Shyr et al., who compared the long-term outcomes of 85 RPDs and 81 open PDs, found the former had improved one- (82.9% vs. 63.8%), three- (45.3% vs. 26.2%) and five-year (26.8% vs. 17.4%) survival rates (P=0.004). However, these findings will have been heavily influenced by selection bias (24).
Aziz et al. (25) (n=11,218) compared the three approaches and found rates of surgical site infection (SSI) were lowest in LPD patients and highest in open patients (3.2% vs. 5.5% vs. 9.0%, P<0.01). Respiratory tract infection was lowest in the RPD group, and highest in the open group (0.9% vs. 3.6% vs. 4.4%, P=0.04). Operation time was longest in the LPD group and shortest in the open group (482 vs. 463 vs. 354 min, P<0.001), and 30-day mortality was lowest in the open group and highest in the LPD group (2.3% vs. 3.3% vs. 3.6%, P=0.02). Long-term outcomes were not studied and the authors concluded that smaller incisions did not improve outcomes. In a recent MA, Aiolfi et al. (26) also compared the three approaches (41 studies, n=56,440). Peri-operative mortality and major morbidity rates were similar. Compared to an open approach, both LPD and RPD were associated with reduced peri-operative blood loss, reduced overall morbidity, shorter length of stay, and reduced rate of readmission (26). The authors advised that the choice of approach should be guided by the skillset of the surgeon but that minimally invasive approaches may improve outcomes (26).
In conclusion, LPD has equivalent short- and long-term outcomes when compared with open PD. However, it is only possible in selected patients and, despite the long learning curve, benefits are likely to be modest. When compared with an open approach, it may be that RPD results in improved histopathological outcomes at the expense of longer operating times and increased financial cost. Whilst selected authors have safety concerns regarding minimally invasive approaches, it is important to remember that safety profile during the application of these techniques is highly dependent on the experience and expertise of the operating surgeon and the centre within which they work.
Type of pancreatic anastomosis
Following resection of the pancreatic head and duodenum, it is necessary to anastomose the pancreatic remnant to a loop of small bowel or the stomach to ensure pancreatic juice reaches the intestinal lumen and the risk of postoperative pancreatic fistula (POPF) is minimised. Some surgeons prefer a pancreatico-jejunostomy (PJ) whereas others prefer a pancreatico-gastrostomy (PG), both are accepted practice (Figure 2). Numerous techniques for forming a PJ have been described. The most recent position statement by the International Study Group of Pancreatic Surgery (ISGPS) does not endorse any particular technique but advises an invaginating approach in patients with a soft pancreas (27). This is supported by the findings of Cao et al. (28) who, in recent MA, found that invaginating techniques were associated with reduced rates of grade B/C POPF (7.4% vs. 12.4%, P=0.006). PG is a reasonable alternative to PJ. Whilst not backed-up by high-quality evidence, some authors claim this method is preferable since pancreatic enzymes are not activated in the acidic stomach, the stomach has a rich blood supply to support the anastomosis, and the join itself is not put under tension (29). Others argue that a PG may be less technically challenging, especially in a patient with a soft pancreas (30).
Multiple MAs have compared PJ and PG outcomes and these have come to conflicting conclusions, possibly due to the high degree of heterogeneity between included studies. Menahem et al. (31) (seven RCTs, n=1,121) found PG reduced the risk of POPF (11.2% vs. 18.7%, P=0.0003), but only four studies used the standardised ISGPS definition. Zhou et al. (32) (six RCTs, n=1,005) reached the same conclusion (OR =0.58; P=0.001). In contrast, Wang et al. (16 RCTs, n=2,396), Daamen et al. (six RCTs, n=1,086), and Jin et al. (11 RCTs, n=1,765) showed PG was not superior to PJ in terms of POPF risk (33-35). Ratnayake et al. (29) (15 RCTs, n=2,428), who compared and ranked five anastomosis techniques, found a PG duct-to-mucosa approach was associated with the lowest rate of clinically relevant POPF.
Concerning other short-term outcomes, Ratnayake et al. (29) found a PG duct-to-mucosa approach was associated with the lowest rates of intra-operative blood transfusion, DGE and intra-abdominal collection. This technique also had the shortest operation times and length of stay, and the lowest overall morbidity and mortality rates (29). Furthermore, Zhou et al. (32) found intra-abdominal collection (OR =0.43; P<0.001) and biliary fistula (OR =0.28; P=0.01) were less common in PG patients. However, Jin et al. (35) found PG patients more commonly experienced post-operative haemorrhage (OR =1.47; P=0.03).
More recently, the Blumgart-style PJ has gained popularity. This technique utilises full thickness transpancreatic sutures to invaginate the jejunum and encapsulate the pancreatic parenchyma. This is thought to reduce the tension on the anastomosis and reduce the risk of a capsule tear. Double sutures are placed at the six and twelve o’clock positions and single sutures are placed at the three and nine o’clock positions. A recent MA by Li et al. (36) (11 studies) compared Blumgart-style PJ (n=1,155) to non-Blumgart PJ (n=1,257) and found the former was associated with reduced risk of grade B/C POPF (OR =0.38; P=0.004).
In conclusion, both PJ and PG are safe and accepted techniques used to fashion a pancreatic anastomosis following pancreatic head resection. PG is arguably less technically challenging and is associated with reduced risk of POPF. It may be that PG is associated with higher rates of postoperative haemorrhage. The impact of anastomosis type on long-term survival is unknown.
Concomitant vascular resection
PDAC commonly invades into the retroperitoneal space, where it can infiltrate the portal vein (PV) and/or the superior mesenteric vein (SMV). Whilst this is no longer an absolute contraindication to PD, the benefits of venous resection (VR) remain controversial. In a recent MA, Wang et al. (37) [41 studies, n=7,567, arterial resection (AR) cases excluded] found VR was associated with increased operation time (491 vs. 399 min, P<0.0001), intra-operative blood loss (929 vs. 581 mL, P=0.0001), risk of post-operative haemorrhage (rates not specified, P<0.0001), risk of DGE (rates not specified, P=0.03), and rate of re-operation (12.3% vs. 11.0%, P=0.008). Interestingly, VR was associated with a lower risk of POPF (7.9% vs. 10.7%, P=0.001) and overall morbidity rates were similar (37). Thirty-day mortality was marginally higher (3.84% vs. 3.17%, P=0.03) in the VR group but 90-day mortality rates were similar (37). Tumour size was significantly larger in the VR patients (35.7 vs. 30.8 mm, P<0.0001), and reduced rate of R0 resection was observed in this group (60.5 vs. 68.7, p<0.0001) (37). One- (RR =0.86; P=0.0009) and five-year (RR =0.64; P=0.004) survival were significantly shorter in the VR group (37). The VR patients likely had more advanced disease although this was not studied. The authors concluded that VR is safe and feasible, and, given the benefit of a R0 resection on OS, it may be necessary for the purpose of achieving a radical resection.
In another recent MA, Peng et al. (38) (30 studies, n=12,031) also found VR was associated with longer operation times (mean difference: 69 min; P<0.0001), increased intra-operative blood loss (mean difference: 202 mL; P<0.0001), larger tumour size (mean difference: 2.43 mm; P<0.0001), and a lower rate of R0 resection (OR =0.64; P<0.0001). Overall morbidity rates, including POPF, were similar but VR was associated with higher rates of bile leak (OR =4.45; P=0.0003), reoperation (OR =1.56; P=0.0001), DGE (OR =1.36; P=0.02), and post-operative haemorrhage (OR =2.18, P<0.0001) (38). VR did not affect length of stay, but was associated with higher inpatient (OR =1.71; P=0.01) and 30-day mortality (OR =2.02, P<0.0001) (38). The authors concluded that VR is associated with considerable additional risk and that it is indicated only in selected cases. They also concluded that, although VR is associated with reduced OS, this likely reflects tumour, rather than intra-operative, factors (38). Some authors have speculated that the length of resected vein is significant. Pan et al. (39) (n=118) studied PD patients who underwent resection of a named vein (SMV or PV) and found VR did not affect OS. However, patients who had >3 cm of vein resected had worse OS (11 vs. 18 months, P=0.02) (39).
AR is associated with significant additional risk. As such, most centres are reluctant to perform PD where there is arterial involvement since outcomes are poor. However, as neoadjuvant therapy (NAT) is now standard of care in patients with borderline resectable and locally advanced disease, this may increase the number of potential surgical candidates in this subgroup. In a recent MA, Rebelo et al. (40) (31 studies, n=7,111, VR cases excluded), showed AR (coeliac artery ± superior mesenteric artery ± common hepatic artery) was associated with higher rates of POPF (27% vs. 14%, P<0.001), DGE (19% vs. 13%, P<0.001), reoperation (11% vs. 4.6%, P<0.001), and peri-operative mortality (5.3% vs. 1.1%, P<0.001). AR was also associated with lower R0 resection rate (73% vs. 80%, P<0.001) and reduced OS (21.9 vs. 45.7 months, P=0.008) (40). Again, the authors concluded that the impact on survival likely reflected tumour factors, and that the need for AR should not be an absolute contraindication to PD (40).
In summary, PD with concomitant vascular resection is associated with additional risk but this should not be an absolute contra-indication to resection. The number of potential surgical candidates with vascular involvement will likely rise due to the increased use of NAT.
Pancreas texture
Intra-operatively, the surgeon will often characterise the texture of the pancreas as either “soft” or “hard”. This is subjective, but it can be a useful predictor of post-operative outcomes. Marchegiani et al. (41) suggested that assessment of pancreatic stiffness using a durometer may be more consistent than using an individual surgeon’s subjective assessment. Martin et al. (42) (n=9,366) found patients with a soft pancreas texture had significantly higher rates of POPF (37% vs. 10%, P<0.001). These findings are supported by other recent studies (43-45). This is likely due to the fragility of the parenchyma and the secretion of high volumes of pancreatic juice (46).
Although the association between a soft pancreas and POPF is well known, this can be difficult to assess pre-operatively using non-invasive methods. Harada et al. (47) (n=16) found liver fibrosis index correlates with pancreatic fibrosis (P=0.018) and POPF (P=0.045), and that real time tissue elastography evaluation of pancreatic stiffness may be a useful predictor of POPF. Shi et al. (48) suggested that pre-operative magnetic resonance imaging (MRI) findings may also be useful for this purpose.
Evidence of pancreatitis
Chronic pancreatitis results in fibrosis which stiffens the parenchyma. As discussed above, a hard pancreas texture is associated with reduced incidence of POPF since the anastomosis is usually less challenging to fashion and less pancreatic juice is secreted (49). Furthermore, chronic pancreatitis may be associated with duct dilatation which further aids the surgeon (see below). Schmidt et al. (50) (n=510, all pathologies) found those with a histological diagnosis of pancreatitis were significantly less likely to develop POPF than those with a malignant diagnosis (9.0% vs. 28%, P=0.02).
Dilatation of the pancreatic duct
A dilated pancreatic duct has long been associated with reduced incidence of POPF. In the Martin et al. (42) study mentioned above, patients were categorised by duct diameter (<3, 3–6 or >6 mm). Patients with duct size of <3 mm were highest risk and those with a duct >6 mm were lowest risk (35.9% vs. 10.1%, P<0.0001). DI Martino et al. (51) (n=107) also concluded that patients who developed POPF had significantly smaller pancreatic duct diameters (2.8 vs. 4.0 mm, P=0.012).
As with pancreas texture, prior authors have attempted to identify predictors of POPF using pre-operative imaging of the main pancreatic duct. Barbier et al. (52) (n=186) found median duct size on pre-operative computed tomography (CT) was significantly smaller in patients who developed a fistula (3.0 vs. 4.5 mm, P<0.01). Ratio of pancreas body thickness to main pancreatic duct size was also higher in POPF patients (5.7 vs. 3.4, P=0.04), and a value >3.8 was associated with increased rates of post-operative haemorrhage (OR =4.3; P=0.01) and reintervention (OR =3.4; P=0.02) (52).
Peri-operative blood transfusion
A number of studies have investigated the correlation between intra-operative blood transfusion and short-term PD outcomes. Dosch et al. (53) (n=6,869) found patients who received an intra- or peri-operative blood transfusion (within 72 h of surgery) were significantly more likely to experience infective complications (34.7% vs. 26.5%, P<0.001). This included SSI, urinary tract infection (UTI), pneumonia and sepsis. Zhang et al. (54) (n=212) reached the same conclusion (OR =3.2; P<0.01). After the exclusion of patients with POPF, blood transfusion remained an independent risk factor for serious infection (OR =5.8; P<0.01). The authors suggested that peri-operative blood transfusion rate should be considered a quality indicator for the performance of PD (54). Hallet et al. (55) (n=17,523) also reached similar conclusions. In this study, peri-operative blood transfusion was associated with increased major morbidity (25.3 vs. 11.3, P<0.0001), length of stay (RR =1.29; P<0.0001) and mortality (5.6% vs. 1.0%, P<0.0001).
The association between peri-operative blood transfusion and infective complications is well documented. This phenomenon is known as transfusion-related immunomodulation (TRIM); it was first described in the late 1980s when renal transplant patients were found to be less likely to reject recipient organs if they had received a peri-operative transfusion. Whilst the exact underlying mechanism is unknown, it is thought the suppression of natural killer cells, T-cells, and neutrophils plays an important role (53). Since PD is a high-risk operation and infective complications are common, further studies are required to investigate the factors which affect peri-operative blood transfusion rates in order to minimise the number of transfusions given.
It has previously been hypothesised that immunosuppression induced by peri-operative blood transfusion may reduce host response to tumour cells and affect OS. This is difficult to investigate due to the impact of confounding factors and remains controversial. Concerning long-term outcomes, Clark et al. (56) (n=170, all pathologies) found peri-operative blood transfusion was not a predictor of OS. In contrast, Abe et al. (57) (n=148, PDAC only) found patients who received a peri-operative blood transfusion had significantly reduced survival at two (3.03% vs. 48.7%) and six (0.0% vs. 10.4%) years (P<0.001). However, the patients who did not receive a transfusion were younger (P=0.029), had higher pre-operative haemoglobin levels (P<0.001), less advanced disease (P=0.001), shorter operation times (P<0.001), were less likely to undergo concomitant vascular resection (P<0.001), and were more likely to achieve a R0 resection (P=0.029) (57). Additional authors have reached similar conclusions (58,59).
Peri-operative blood transfusion rate is often considered non-modifiable but it is arguably modifiable. For example, individual anaesthetists may have differing opinions on transfusion thresholds and individual units may have differing transfusion protocols. It has been hypothesised that one of the reasons larger centres have superior PD outcomes is that these units tend to have lower thresholds for transfusion and increased use of reserved blood units relative to the number given. This was investigated by Lammi et al. (60) (n=1,337, total pancreatectomies also included) and no differences were observed between high-, medium- and low-volume centres in terms of blood usage, transfusion trigger point, or use of reserved units. However, during the study period (2002–2011), the trigger points decreased (P=0.003) and the usage of reserved units increased (P<0.001) at high-volume centres relative to the other units.
Due to the potential impact of peri-operative blood transfusion, a number of authors have investigated the effect of estimated intra-operative blood loss (EBL) on PD outcomes. EBL is known to be subjective and imprecise, and hence its use in studies if often criticised. Ghee et al. (61) recently found that surgeons tend to significantly underestimate EBL (P=0.009) whereas anaesthetists tend to overestimate (P=0.004). Seykora et al. (62) (n=5,323) categorised EBL into 0–300, 301–750, 751–1,300 and >1,300 mL, and found median EBL to be 400 mL. Intra- and post-operative transfusion rates were 15.8% and 24.8%, respectively. Progressive EBL corelated with intra- but not post-operative transfusion in a dose-dependent manner (P<0.0001) and was associated with poor peri-operative outcomes (62). Hence, the authors concluded that efforts should be made to minimize intra-operative blood loss and that there are gains to be made by targeting modifiable factors (62). Furthermore, Casciani et al. (63) (n=7,706) matched 966 PD patients with EBL ≤700 mL to 966 with EBL >700 mL. The former had lower rates of clinically relevant POPF (19.4% vs. 12.6%), major morbidity (27.8% vs. 15.6%), transfusion (50.1% vs. 14.3%), reoperation (9.2% vs. 4.0%) and 90-day mortality (4.7% vs. 2.0%, all P<0.001). The authors suggested that blood loss should be minimised by careful transection of the pancreatic neck using transfusion sutures to control the pancreatic arcades, using electrocautery to dissect the parenchyma, and to combat any pulsatile bleeding with sutures (63). In addition, they advised an “artery first” approach when dissecting the pancreatic head from the mesenteric axis to allow early detection of gross vascular infiltration. They go on to conclude that operative techniques associated with reduced EBL may be preferrable as this may reduce the need for transfusion e.g., a PG rather than a PJ anastomosis, and the use of externalised trans-anastomotic stents, transperitoneal drainage, and prophylactic octreotide (63).
In conclusion, PD patients who receive a peri-operative blood transfusion appear to be at higher risk of developing infective complications. Whether transfusion affects long-term outcomes remains controversial as it is difficult to exclude confounding variables, but it may be associated with reduced OS. Where suitable, pre-operative anaemia should be corrected and efforts should be made to minimise intra-operative blood loss.
Discussion
Myriad intra-operative factors are known to affect PD outcomes (Table 1). Whilst some are non-modifiable, an appreciation for these allows informed assessment of potential surgical candidates and guides risk-benefit discussions. Patients with ASA grade III are high-risk and have poor short- and long-term outcomes. Patients should be optimised prior to surgery where possible and the appropriate members of the mutli-disciplinary team should be involved early so that the best possible outcome can be achieved. Whilst advanced age should never be an absolute contra-indication to PD, one should be very cautious when offering resection to a patient over 75-year-old with an ASA grade of III, as this sub-group have additional associated risks. These patients should be made aware of their additional risk prior to being consented. Further important non-modifiable factors include soft pancreas texture, absence of pancreatitis and small main pancreatic duct. Each of these is associated with an increased risk POPF. Surgeons may wish to adapt their practice in patients with these characteristics in order to optimise their outcomes.
Table 1
| Intra-/peri-operative factors | Risk of POPF | Risk of infection | Risk of DGE | Risk of post-operative haemorrhage | Operation time | Intra-operative blood loss | Length of stay | Peri-operative morbidity | Peri-operative mortality | OS |
|---|---|---|---|---|---|---|---|---|---|---|
| ASA grade III (vs. ASA grade I/II) | ↑ | ↑ | ↓ | |||||||
| PP (vs. pylorus-resecting) technique | ↑ | ↓ | ↓ | |||||||
| Laparoscopic (vs. open) approach | ↑ | ↓ | ↓ | ↑ | ||||||
| Robotic (vs. open) approach | ↑ | ↓ | ↑ | |||||||
| PG (vs. PJ) anastomosis | ↓ | ↑ | ↑ | ↓ | ↓ | |||||
| Concomitant VR | ↓ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | |||
| >3 cm vein resection | ↓ | |||||||||
| Concomitant AR | ↑ | ↑ | ↑ | ↑ | ↓ | |||||
| Soft pancreas texture | ↑ | |||||||||
| Evidence of pancreatitis | ↓ | |||||||||
| Main pancreatic duct >6 mm diameter | ↓ | |||||||||
| Peri-operative blood transfusion | ↑ | / | ↑ | ↑ | ↓ |
Increased or decreased risk/overall survival compared to patients without the factor. References within the article text. Many of the effects demonstrated are likely influenced by selection bias and/or confounding variables. PD, pancreaticoduodenectomy; POPF, postoperative pancreatic fistula; DGE, delayed gastric emptying; OS, overall survival; ASA, American Society of Anesthesiologists; PP, pylorus-preserving; PG, pancreatico-gastrostomy; PJ, pancreatico-jejunostomy; VR, venous resection; AR, arterial resection.
Many of the modifiable intra-operative risk factors discussed relate to surgical technique and approach. A pylorus-preserving technique may reduce operation times and intra-operative blood loss, whilst achieving comparable oncological outcomes. However, this likely results in higher rates of DGE. Minimally invasive techniques have become more popular in recent years but an open approach remains the standard of care. Some studies have demonstrated superior outcomes following LPD and/or RPD but these are likely affected by selection bias and these procedures are only available in certain units.
PG and PJ are both acceptable and numerous studies have compared the two techniques. It may be that PG is associated with reduced blood transfusion rate and operation time, at the expense of increased risk of post-operative haemorrhage. As with any surgical technique, it is important to weigh this up against the expertise of the surgeon performing the procedure and the team within which they work. Concomitant vascular resection remains a controversial topic. This is associated with increased peri-operative morbidity and evidence supporting improved long-term outcomes is lacking. Whilst those who undergo concomitant vascular resection have reduced OS, this likely reflects tumour factors and the number of patients with vascular involvement who are appropriate surgical candidates is set to rise with increased use of NAT.
Peri-operative blood transfusion rate is arguably a modifiable factor. Those who receive a transfusion are more likely to experience an infective complication and may have worse long-term outcomes. Whilst these findings are likely influenced by confounding variables, some authors suggest peri-operative transfusion rate should be a performance indicator for PD and argue surgeons should alter their approach where appropriate to minimise blood loss.
The factors discussed in this review were pre-selected and are currently being investigated by the Recurrence After Whipple’s (RAW) study. This is an international, multi-centre, retrospective, analysis which aims to assess the impact of these variables on patterns of recurrence and surgical outcomes following PD (NCT04596865). Results are expected in 2022. Much of the evidence on the factors discussed is limited to small studies or those influenced by selection bias or confounding variables. Hence, we argue a robust study is required so that models can be created which can estimate risk in individual patients.
This article aims to provide the reader with a broad overview and has not attempted to answer a specific research question. We acknowledge that we have not considered all intra-operative factors which affect PD outcomes and, whilst we have attempted to summarise the most relevant studies and consolidate the recent evidence, we have not included all relevant studies.
Conclusions
PD remains a high-risk operation associated with considerable morbidity. In the absence of surgical complications, few patients achieve long-term survival due to disease recurrence, so efforts should be made to optimise outcomes wherever feasible. A number of intra-operative variables affect short- and long-term PD outcomes. Given the limitations of the current literature, a robust study is required which considers confounding variables. A greater understanding of these variables will improve patient selection, guide risk-benefit discussions and allow surgeons to adjust their own practice to improve outcomes.
Acknowledgments
We would like to thank John Peter Ovens for providing the illustrations for Figures 1,2.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://apc.amegroups.com/article/view/10.21037/apc-21-16/rc
Peer Review File: Available at https://apc.amegroups.com/article/view/10.21037/apc-21-16/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-21-16/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019;10:10-27. [Crossref] [PubMed]
- Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J Anaesth 2011;55:111-5. [Crossref] [PubMed]
- Tiret L, Hatton F, Desmonts JM, et al. Prediction of outcome of anaesthesia in patients over 40 years: a multifactorial risk index. Stat Med 1988;7:947-54. [Crossref] [PubMed]
- Eeson G, Chang N, McGahan CE, et al. Determination of factors predictive of outcome for patients undergoing a pancreaticoduodenectomy of pancreatic head ductal adenocarcinomas. HPB (Oxford) 2012;14:310-6. [Crossref] [PubMed]
- Braga M, Capretti G, Pecorelli N, et al. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg 2011;254:702-7; discussion 707-8. [Crossref] [PubMed]
- Wiltberger G, Muhl B, Benzing C, et al. Preoperative risk stratification for major complications following pancreaticoduodenectomy: Identification of high-risk patients. Int J Surg 2016;31:33-9. [Crossref] [PubMed]
- Nikfarjam M. Pylorus preserving pancreaticoduodenectomy. Saudi J Gastroenterol 2010;16:65. [Crossref] [PubMed]
- Klaiber U, Probst P, Büchler MW, et al. Pylorus preservation pancreatectomy or not. Transl Gastroenterol Hepatol 2017;2:100. [Crossref] [PubMed]
- Hüttner FJ, Fitzmaurice C, Schwarzer G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2016;2:CD006053. [Crossref] [PubMed]
- Diener MK, Fitzmaurice C, Schwarzer G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2014;CD006053. [PubMed]
- Yang C, Wu HS, Chen XL, et al. Pylorus-preserving versus pylorus-resecting pancreaticoduodenectomy for periampullary and pancreatic carcinoma: a meta-analysis. PLoS One 2014;9:e90316. [Crossref] [PubMed]
- Zhou Y, Lin L, Wu L, et al. A case-matched comparison and meta-analysis comparing pylorus-resecting pancreaticoduodenectomy with pylorus-preserving pancreaticoduodenectomy for the incidence of postoperative delayed gastric emptying. HPB (Oxford) 2015;17:337-43. [Crossref] [PubMed]
- Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. [Crossref] [PubMed]
- Umemura A, Nitta H, Takahara T, et al. Current status of laparoscopic pancreaticoduodenectomy and pancreatectomy. Asian J Surg 2018;41:106-14. [Crossref] [PubMed]
- Boggi U, Amorese G, Vistoli F, et al. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc 2015;29:9-23. [Crossref] [PubMed]
- Nickel F, Haney CM, Kowalewski KF, et al. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg 2020;271:54-66. [Crossref] [PubMed]
- Wang M, Li D, Chen R, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2021;6:438-47. [Crossref] [PubMed]
- Mazzola M, Giani A, Crippa J, et al. Totally laparoscopic versus open pancreaticoduodenectomy: A propensity score matching analysis of short-term outcomes. Eur J Surg Oncol 2021;47:674-80. [Crossref] [PubMed]
- Chen K, Pan Y, Huang CJ, et al. Laparoscopic versus open pancreatic resection for ductal adenocarcinoma: separate propensity score matching analyses of distal pancreatectomy and pancreaticoduodenectomy. BMC Cancer 2021;21:382. [Crossref] [PubMed]
- Ausania F, Landi F, Martínez-Pérez A, et al. A meta-analysis of randomized controlled trials comparing laparoscopic vs open pancreaticoduodenectomy. HPB (Oxford) 2019;21:1613-20. [Crossref] [PubMed]
- van Hilst J, de Rooij T, Bosscha K, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 2019;4:199-207. [Crossref] [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [Crossref] [PubMed]
- Da Dong X, Felsenreich DM, Gogna S, et al. Robotic pancreaticoduodenectomy provides better histopathological outcomes as compared to its open counterpart: a meta-analysis. Sci Rep 2021;11:3774. [Crossref] [PubMed]
- Shyr YM, Wang SE, Chen SC, et al. Robotic pancreaticoduodenectomy for pancreatic head cancer and periampullary lesions. Ann Gastroenterol Surg 2021;5:589-96. [Crossref] [PubMed]
- Aziz H, Shahjehan F, Jie T, et al. Analysis of Outcomes of Open, Robotic and Laparoscopic Pancreaticoduodenectomy Using NSQIP. J Pancreas 2018;19:291-5.
- Aiolfi A, Lombardo F, Bonitta G, et al. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg 2021;73:909-22. [Crossref] [PubMed]
- Shrikhande SV, Sivasanker M, Vollmer CM, et al. Pancreatic anastomosis after pancreatoduodenectomy: A position statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2017;161:1221-34. [Crossref] [PubMed]
- Cao Z, Luo W, Qiu J, et al. Is Invagination Anastomosis More Effective in Reducing Clinically Relevant Pancreatic Fistula for Soft Pancreas After Pancreaticoduodenectomy Under Novel Fistula Criteria: A Systematic Review and Meta-Analysis. Front Oncol 2020;10:1637. [Crossref] [PubMed]
- Ratnayake CBB, Wells CI, Kamarajah SK, et al. Critical appraisal of the techniques of pancreatic anastomosis following pancreaticoduodenectomy: A network meta-analysis. Int J Surg 2020;73:72-7. [Crossref] [PubMed]
- Keck T, Wellner UF, Bahra M, et al. Pancreatogastrostomy Versus Pancreatojejunostomy for RECOnstruction After PANCreatoduodenectomy (RECOPANC, DRKS 00000767): Perioperative and Long-term Results of a Multicenter Randomized Controlled Trial. Ann Surg 2016;263:440-9. [Crossref] [PubMed]
- Menahem B, Guittet L, Mulliri A, et al. Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Ann Surg 2015;261:882-7. [Crossref] [PubMed]
- Zhou Y, Yu J, Wu L, et al. Meta-analysis of pancreaticogastrostomy versus pancreaticojejunostomy on occurrences of postoperative pancreatic fistula after pancreaticoduodenectomy. Asian J Surg 2015;38:155-60. [Crossref] [PubMed]
- Wang W, Zhang Z, Gu C, et al. The optimal choice for pancreatic anastomosis after pancreaticoduodenectomy: A network meta-analysis of randomized control trials. Int J Surg 2018;57:111-6. [Crossref] [PubMed]
- Daamen LA, Smits FJ, Besselink MG, et al. A web-based overview, systematic review and meta-analysis of pancreatic anastomosis techniques following pancreatoduodenectomy. HPB (Oxford) 2018;20:777-85. [Crossref] [PubMed]
- Jin Y, Feng YY, Qi XG, et al. Pancreatogastrostomy vs pancreatojejunostomy after pancreaticoduodenectomy: An updated meta-analysis of RCTs and our experience. World J Gastrointest Surg 2019;11:322-32. [Crossref] [PubMed]
- Li Z, Wei A, Xia N, et al. Blumgart anastomosis reduces the incidence of pancreatic fistula after pancreaticoduodenectomy: a systematic review and meta-analysis. Sci Rep 2020;10:17896. [Crossref] [PubMed]
- Wang X, Demir IE, Schorn S, et al. Venous resection during pancreatectomy for pancreatic cancer: a systematic review. Transl Gastroenterol Hepatol 2019;4:46. [Crossref] [PubMed]
- Peng C, Zhou D, Meng L, et al. The value of combined vein resection in pancreaticoduodenectomy for pancreatic head carcinoma: a meta-analysis. BMC Surg 2019;19:84. [Crossref] [PubMed]
- Pan G, Xie KL, Wu H. Vascular resection in pancreatic adenocarcinoma with portal or superior mesenteric vein invasion. World J Gastroenterol 2013;19:8740-4. [Crossref] [PubMed]
- Rebelo A, Büdeyri I, Heckler M, et al. Systematic review and meta-analysis of contemporary pancreas surgery with arterial resection. Langenbecks Arch Surg 2020;405:903-19. [Crossref] [PubMed]
- Marchegiani G, Ballarin R, Malleo G, et al. Quantitative Assessment of Pancreatic Texture Using a Durometer: A New Tool to Predict the Risk of Developing a Postoperative Fistula. World J Surg 2017;41:2876-83. [Crossref] [PubMed]
- Martin AN, Narayanan S, Turrentine FE, et al. Pancreatic duct size and gland texture are associated with pancreatic fistula after pancreaticoduodenectomy but not after distal pancreatectomy. PLoS One 2018;13:e0203841. [Crossref] [PubMed]
- Jakhmola CK, Kumar A. Whipple's pancreaticoduodenectomy: Outcomes at a tertiary care hospital. Med J Armed Forces India 2014;70:321-6. [Crossref] [PubMed]
- Ke ZX, Xiong JX, Hu J, et al. Risk Factors and Management of Postoperative Pancreatic Fistula Following Pancreaticoduodenectomy: Single-center Experience. Curr Med Sci 2019;39:1009-18. [Crossref] [PubMed]
- Schuh F, Mihaljevic AL, Probst P, et al. A Simple Classification Of Pancreatic Duct Size and Texture Predicts Postoperative Pancreatic Fistula: A classification of the International Study Group of Pancreatic Surgery (ISGPS). Ann Surg 2021; Epub ahead of print. [Crossref] [PubMed]
- Conzo G, Gambardella C, Tartaglia E, et al. Pancreatic fistula following pancreatoduodenectomy. Evaluation of different surgical approaches in the management of pancreatic stump. Literature review. Int J Surg 2015;21:S4-9. [Crossref] [PubMed]
- Harada N, Yoshizumi T, Maeda T, et al. Preoperative Pancreatic Stiffness by Real-time Tissue Elastography to Predict Pancreatic Fistula After Pancreaticoduodenectomy. Anticancer Res 2017;37:1909-15. [Crossref] [PubMed]
- Shi Y, Liu Y, Gao F, et al. Pancreatic Stiffness Quantified with MR Elastography: Relationship to Postoperative Pancreatic Fistula after Pancreaticoenteric Anastomosis. Radiology 2018;288:476-84. [Crossref] [PubMed]
- Machado NO. Pancreatic fistula after pancreatectomy: definitions, risk factors, preventive measures, and management-review. Int J Surg Oncol 2012;2012:602478. [PubMed]
- Schmidt CM, Choi J, Powell ES, et al. Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg 2009;2009:404520. [Crossref] [PubMed]
- DI Martino M. Predictive Factors of Pancreatic Fistula After Pancreaticoduodenectomy and External Validation of Predictive Scores. Anticancer Res 2019;39:499-504. [Crossref] [PubMed]
- Barbier L, Mège D, Reyre A, et al. Predict pancreatic fistula after pancreaticoduodenectomy: ratio body thickness/main duct. ANZ J Surg 2018;88:E451-5. [Crossref] [PubMed]
- Dosch AR, Grigorian A, Delaplain PT, et al. Perioperative blood transfusion is associated with an increased risk for post-surgical infection following pancreaticoduodenectomy. HPB (Oxford) 2019;21:1577-84. [Crossref] [PubMed]
- Zhang L, Liao Q, Zhang T, et al. Blood Transfusion is an Independent Risk Factor for Postoperative Serious Infectious Complications After Pancreaticoduodenectomy. World J Surg 2016;40:2507-12. [Crossref] [PubMed]
- Hallet J, Mahar AL, Tsang ME, et al. The impact of peri-operative blood transfusions on post-pancreatectomy short-term outcomes: an analysis from the American College of Surgeons National Surgical Quality Improvement Program. HPB (Oxford) 2015;17:975-82. [Crossref] [PubMed]
- Clark E, Connor S, Taylor MA, et al. Perioperative transfusion for pancreaticoduodenectomy and its impact on prognosis in resected pancreatic ductal adenocarcinoma. HPB (Oxford) 2007;9:472-7. [Crossref] [PubMed]
- Abe T, Amano H, Hanada K, et al. Perioperative Red Blood Cell Transfusion Is Associated with Poor Long-term Survival in Pancreatic Adenocarcinoma. Anticancer Res 2017;37:5863-70. [PubMed]
- Park HM, Park SJ, Shim JR, et al. Perioperative transfusion in pancreatoduodenectomy: The double-edged sword of pancreatic surgeons. Medicine (Baltimore) 2017;96:e9019. [Crossref] [PubMed]
- Sutton JM, Kooby DA, Wilson GC, et al. Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg 2014;18:1575-87. [Crossref] [PubMed]
- Lammi JP, Eskelinen M, Tuimala J, et al. Blood Transfusions in Major Pancreatic Surgery: A 10-Year Cohort Study Including 1404 Patients Undergoing Pancreatic Resections in Finland. Scand J Surg 2019;108:210-5. [Crossref] [PubMed]
- Ghee L, Thomas S, Kowdley G, et al. Measured vs estimated blood loss during pancreaticoduodenectomy and other major abdominal operations: interim analysis. HPB 2017;19:S79. [Crossref]
- Seykora TF, Ecker BL, McMillan MT, et al. The Beneficial Effects of Minimizing Blood Loss in Pancreatoduodenectomy. Ann Surg 2019;270:147-57. [Crossref] [PubMed]
- Casciani F, Trudeau MT, Asbun HJ, et al. The effect of high intraoperative blood loss on pancreatic fistula development after pancreatoduodenectomy: An international, multi-institutional propensity score matched analysis. Surgery 2021;170:1195-204. [Crossref] [PubMed]
Cite this article as: Russell TB, Labib PL, Aroori S. Selected intra-operative factors which affect pancreaticoduodenectomy outcomes: a narrative review. Ann Pancreat Cancer 2022;5:2.


