
Pancreatic neuroendocrine tumor in a 27-year-old patient with Cornelia de Lange syndrome: a case report
Introduction
Cornelia de Lange syndrome (CdLS) is a rare genetic disorder that can result in developmental abnormalities of multiple organs with varying severity (1,2). These include structural abnormalities as well as delayed growth and intellectual disability, with an increased incidence of autism. Distinguishing features include arched eyebrows with synophrys, elongated eyelashes, small and upturned nose, small chin and widely spaced teeth (1). The most common cause is a mutation in the NIPBL gene, which is an integral component of the cohesin complex; SMC1A is another cohesin gene which is less commonly involved (1). While there exist case reports on patients with CdLS developing tumors they do not acknowledge any increased risk of cancer development in these patients (2).
Pancreatic neuroendocrine tumors (PNETs) are rare tumors of the pancreas representing <3% of all pancreatic neoplasms (3). Furthermore, PNETs are less frequent in younger populations with an estimated 0.8 cases reported per 1,000,000 individuals in patients younger than 30 years of age (4). Herein, we report the first case of a patient with CdLS developing a PNET. We present the following article in accordance with the CARE reporting checklist (available at https://apc.amegroups.com/article/view/10.21037/apc-21-12/rc).
Case presentation
The patient was a 27-year-old Caucasian female who presented with epigastric pain. She had a past medical history significant for clinically diagnosed CdLS, polycystic ovarian syndrome (PCOS), gastroesophageal reflux disease (GERD), delayed gastric emptying (DGE), hypertriglyceridemia, and pre-diabetes. Relevant family history was negative for any suggestions of multiple endocrine neoplasia (MEN) or Von Hippel-Lindau (VHL) syndrome. Both her paternal uncle and paternal grandfather were previously diagnosed with colorectal cancer. Whole exome sequencing revealed 32% mosaicism on cheek swab for a de novo pathogenic variant in NIPBL p.S207X, c.620 C>G (blood testing was previously negative) and a likely pathogenic variant maternally inherited in FAN1, a gene involved in DNA repair, p.L925PfsX25, c.2774_2775delTT.
Investigations
To investigate the cause of the new onset epigastric pain, diagnostic imaging was indicated. Initial radiological workup via ultrasound (US) and computed tomography (CT) demonstrated peripancreatic inflammatory changes and a 2.8 cm hypoechoic solid mass in the head of the pancreas with no apparent vascular involvement and a lymph node in the porta hepatis that was suspicious for malignancy (Figure 1). The patient was noted to have mild hepatomegaly with moderate hepatic steatosis. Of note, this tumor lacked the marked vascularity commonly associated with PNETs.
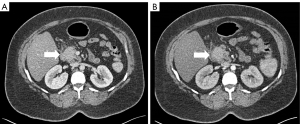
Subsequent endoscopic US and fine needle aspiration demonstrate a well-differentiated, low-grade PNET and the suspicious lymph node was found to be benign.
Differential diagnosis
Following diagnostic imaging, a working diagnosis of a PNET was established. In accordance with the results of the endoscopic biopsy, the patient was diagnosed with a PNET. The patient was recommended surgical resection.
Treatment
Six months after diagnosis, the patient underwent an uneventful surgical resection by means of a pancreatoduodenectomy.
Outcome and follow-up
Final histopathologic evaluation yielded a 3.0 cm well-differentiated, non-functional PNET with intermediate lymphovascular invasion, no perineural invasion, and negative margins. A total of 3 out of 16 harvested lymph nodes were positive for metastatic disease. The tumor was found to have a Ki-67 index of 1% with a mitotic rate of <1 mitoses/10 high power fields (HPF). The tumor extended beyond the pancreas into the duodenum and was therefore staged as stage IIB disease (T2N1M0) (5). The postoperative course was uneventful with the exception of a brief episode of tachycardia and low grade fever, both of which quickly resolved with conservative non-interventional management and the patient was discharged in stable condition.
At a 90-day follow-up the patient was recovering well and was recommended routine imaging based surveillance. Her incision was well-healed, blood-sugars controlled off oral medications and insulin, and plans were made to stop pancreatic enzyme supplementation.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
CdLS is a rare genetic condition that results in abnormal growth, intellectual disability and multiple developmental anomalies most commonly affecting the cardiac, gastrointestinal, and craniofacial systems. Intellectual impairment ranges from mild to severe (1). The incidence is estimated to be 1 in 10,000 to 1 in 30,000 newborns in the United States (6). In rare cases, patients with CdLS have been reported to develop malignant tumors however, literature is limited to case reports (7). In a multi-institutional retrospective review, Schrier et al. reported a single case of lymphoma, three cases of esophageal cancer, and one case of gastric cancer in patients with CdLS (8). Two patients with CdLS have been reported to have developed Wilms tumors (WT). Santoro et al. described a case of a 4-year-old female who was incidentally diagnosed with a WT upon US examination (9). The other case, detailed by Maruiwa et al. described a post-mortem finding of a WT in a seven-month-old female who died because of bronchopneumonia (10). Additional individual cases of patients with CdLS with malignant tumors included one developing an esophageal adenocarcinoma and the other developing an endometrial carcinoma (11,12). There is also a report of a benign tumor in a patient with CdLS manifesting as a teratoma (13). To the best of our knowledge, the patient described in this report is the tenth reported case of a malignant tumor in patients with CdLS and the first of pancreatic origin.
From a PNET perspective, this case is unique because while PNETs are rare pancreatic tumors, they are even rarer in younger patients (4). The patient’s young age at the time of diagnosis raises the question whether this rare genetic disorder could have potentially contributed to the development of the tumor. Furthermore, PNETs have also been found, at times, to be associated with multiple other genetic conditions including MEN1, VHL, neurofibromatosis 1 (NF-1), and tuberous sclerosis complex (TSC) (3).
Evidence for premature aging in patients with CdLS is well documented, Kline et al. having reported early development of Barrett esophagus, as well as osteoporosis in teenagers with CdLS (2). Also, premature graying of hair in some CdLS patients further supports this phenomenon (2). Multiple mutations in five genes have been identified in patients with CdLS, some of which are theorized to drive the pathogenesis of PCOS (12). It is suggested that the NIPBL and SMC1A mutations that impair DNA damage repair mechanisms contribute to premature aging and promote tumorigenesis in these patients (1). Indeed, SMC1A is directly associated both with growth of prostate cancer and metastasis of colon cancer (14,15). This patient’s mosaic mutation in NIPBL confirms her clinical diagnosis.
The likely pathogenic mutation in FAN1 may also have some relevance. Homozygous variants have been associated with a type of interstitial nephritis (karyomegalic), in which the kidney, but also other organs, has enlarged nuclei of the cells, interstitial infiltrations and fibrosis (16). Heterozygous variants have been seen in families with hereditary nonpolyposis colorectal cancer and may predispose to this type of cancer (17). Of greatest interest, FAN1 was identified to be a possible susceptibility gene to pancreatic adenocarcinoma (18). Overall, FAN1 appears to act as a tumor suppressor gene, and a likely pathogenic variant with a second hit could have predisposed her to the tumor (19).
Interestingly, the patient presented in this report had a relevant past medical history of GERD and PCOS. This is similar to both GERD and PCOS being reported in the aforementioned patients who developed esophageal adenocarcinoma and endometrial carcinoma respectively (11,12). Consequently, it appears that CdLS may drive certain medical conditions including GERD, Barrett esophagus, and PCOS (7). These conditions can potentially predispose patients to an increased risk of developing malignant tumors of the esophagus, endometrium, kidneys, and pancreas (9,11,12). While the evidence is limited, this report in conjunction with those reported in the past suggest that patients with CdLS might be at an increased risk of developing certain malignant tumors.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apc.amegroups.com/article/view/10.21037/apc-21-12/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-21-12/coif). CLW serves as an unpaid editorial board member of Annals of Pancreatic Cancer from January 2021 to December 2022. AJ serves as the unpaid Section Editor (Surgeons in Training) of Annals of Pancreatic Cancer from May 2021 to April 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boyle MI, Jespersgaard C, Brøndum-Nielsen K, et al. Cornelia de Lange syndrome. Clin Genet 2015;88:1-12. [Crossref] [PubMed]
- Kline AD, Grados M, Sponseller P, et al. Natural history of aging in Cornelia de Lange syndrome. Am J Med Genet C Semin Med Genet 2007;145C:248-60. [Crossref] [PubMed]
- Verbeke CS. Endocrine tumours of the pancreas. Histopathology 2010;56:669-82. [Crossref] [PubMed]
- StatBite. Pancreatic neuroendocrine tumors: incidence by age and sex. J Natl Cancer Inst 2011;103:626. [Crossref] [PubMed]
- Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017;265:185-91.
- Lister Hill National Center for Biomedical Communications. Genetics home reference. Bethesda, MD: National Library of Medicine, National Institutes of Health; 2003.
- Kline AD, Moss JF, Selicorni A, et al. Diagnosis and management of Cornelia de Lange syndrome: first international consensus statement. Nat Rev Genet 2018;19:649-66. [Crossref] [PubMed]
- Schrier SA, Sherer I, Deardorff MA, et al. Causes of death and autopsy findings in a large study cohort of individuals with Cornelia de Lange syndrome and review of the literature. Am J Med Genet A 2011;155A:3007-24. [Crossref] [PubMed]
- Santoro C, Apicella A, Casale F, et al. Unusual association of non-anaplastic Wilms tumor and Cornelia de Lange syndrome: case report. BMC Cancer 2016;16:365. [Crossref] [PubMed]
- Maruiwa M, Nakamura Y, Motomura K, et al. Cornelia de Lange syndrome associated with Wilms' tumour and infantile haemangioendothelioma of the liver: report of two autopsy cases. Virchows Arch A Pathol Anat Histopathol 1988;413:463-8. [Crossref] [PubMed]
- DuVall GA, Walden DT. Adenocarcinoma of the esophagus complicating Cornelia de Lange syndrome. J Clin Gastroenterol 1996;22:131-3. [Crossref] [PubMed]
- Tate K, Yoshida H, Ishikawa M, et al. Endometrial Carcinoma With an Unusual Morphology in a Patient With Cornelia de Lange Syndrome: A Case Study. Int J Gynecol Pathol 2019;38:340-5. [Crossref] [PubMed]
- Banait N, Fenton A, Splitt M. Cornelia de Lange syndrome due to mosaic NIPBL mutation: antenatal presentation with sacrococcygeal teratoma. BMJ Case Rep 2015;2015:bcr2015211006. [Crossref] [PubMed]
- Zhou P, Xiao N, Wang J, et al. SMC1A recruits tumor-associated-fibroblasts (TAFs) and promotes colorectal cancer metastasis. Cancer Lett 2017;385:39-45. [Crossref] [PubMed]
- Pan XW, Gan SS, Ye JQ, et al. SMC1A promotes growth and migration of prostate cancer in vitro and in vivo. Int J Oncol 2016;49:1963-72. [Crossref] [PubMed]
- Zhou W, Otto EA, Cluckey A, et al. FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair. Nat Genet 2012;44:910-5. [Crossref] [PubMed]
- Seguí N, Mina LB, Lázaro C, et al. Germline Mutations in FAN1 Cause Hereditary Colorectal Cancer by Impairing DNA Repair. Gastroenterology 2015;149:563-6. [Crossref] [PubMed]
- Smith AL, Alirezaie N, Connor A, et al. Candidate DNA repair susceptibility genes identified by exome sequencing in high-risk pancreatic cancer. Cancer Lett 2016;370:302-12. [Crossref] [PubMed]
- Lachaud C, Moreno A, Marchesi F, et al. Ubiquitinated Fancd2 recruits Fan1 to stalled replication forks to prevent genome instability. Science 2016;351:846-9. [Crossref] [PubMed]
Cite this article as: Wright MJ, Kline AD, Wolfgang CL, Javed AA. Pancreatic neuroendocrine tumor in a 27-year-old patient with Cornelia de Lange syndrome: a case report. Ann Pancreat Cancer 2022;5:5.


