
A tumor intrinsic role of CD73 in pancreatic adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies due to delayed diagnosis, early metastasis, and limited response to chemotherapy (1,2). Gemcitabine-based chemotherapy remains one of most commonly used treatments for all stages of PDAC (3). PDAC is often refractory to the gemcitabine chemotherapy or, after an initial response, quickly develops the resistance to gemcitabine. Therefore, understanding the molecular mechanisms of chemoresistance is critical for the development of treatment strategies that overcome the resistance to chemotherapy including gemcitabine. In a recently published study, Yu et al. revealed an important role and novel mechanism of CD73 in chemotherapeutic resistance in PDAC (4). This study also tested the potential therapeutic approach that can improve the outcome of the contemporary treatments for PDAC.
CD73 (a.k.a. NT5E) is a glycosylphosphatidylinositol (GPI)-anchored cell surface enzyme that is expressed on multiple cell types, including endothelial cells, subtypes of lymphocytes, stromal cells and selected types of tumor cells (5). Its main function is to generate extracellular adenosine from adenosine monophosphate (AMP) through the coordinated action of CD39 that catalyzes the phosphohydrolysis of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) to AMP (5,6). The balance between ATP and adenosine is crucial to restrict inflammatory responses to prevent uncontrolled tissue damage. Adenosine and ATP are normally present at very low levels in extracellular fluids. In stressful situations such as inflammation, ischemia, and malignancy, high levels of ATP will be released to extracellular fluids, where it functions as a Danger-Associated Molecular Pattern (DAMP) to promote both innate and adaptive immune responses by signaling through purinergic receptors, that is, P2XRs and P2YRs, on a wide range of immune cells (7-9). During these processes, however, extracellular ATP is rapidly hydrolyzed by the enzymatic cascade via CD39 and CD73, culminating in the formation of adenosine (8,10). In contrast to ATP, extracellular adenosine, via interaction with its four G-protein-coupled receptors (A1R, A2AR, A2BR, and A3R), can enhance the suppressive immune cells including myeloid-derived suppressor cells (MDSCs) and T regulatory cells (Tregs) and attenuate the protective immune cells such as T cells and natural killer (NK) cells to dampen immune response (11,12). Thus, CD73, as a rate-limiting enzyme for adenosine production, plays a critical role to protect against excessive immune responses by converting/switching ATP-triggered immune activation to adenosine-mediated immunosuppression (9).
CD73 is highly expressed in most human solid tumors, which is invariably hijacked by tumor microenvironment (TME) to promote and maintain immune evasion (13). Within the TME, besides of tumor cells, a subset of endothelial cells, Tregs, MDSC, NK cells, tumor-associated macrophages (TAMs) and cancer associated fibroblasts (CAFs) also express high level of CD73, which serves as a major immune suppressive mediator in TME mainly through generation of extracellular adenosine (9). As shown in Figure 1, adenosine-mediated activation of A2AR in effector T cell inhibits cell activation and proliferation, and induces T cell anergy (14,15). Besides, A2AR activation also suppresses NK cell maturation, activation, and cytotoxic function (16,17). Moreover, A2AR activation in naive CD4+ T cells promotes their differentiation towards Foxp3+ and LAG-3+ Treg cells (18,19). In addition, CD73-produced adenosine promotes the immune suppressive function and the infiltration of MDSC to tumor mainly through A2BR (20,21). Generally, CD73 impairs anti-tumor immunity by inhibiting the cytotoxic activity of effector cells, while enhancing the function of regulatory immune cells (9). As such, CD73 has mainly been characterized as a promising target for cancer immunotherapy. In preclinical models, targeted blockade of CD73 using either monoclonal antibodies (mAb) or small molecule inhibitor such as APCP (α, β-methylene ADP) have demonstrated encouraging antitumor effects as monotherapy or in combination with immune checkpoint blockade, targeted therapy, or conventional therapy (5,22-25). Emerging evidence indicates that the combination of CD73 inhibitor and immune checkpoint blockade displays promising clinical activity in patients with advanced solid tumors (26,27).
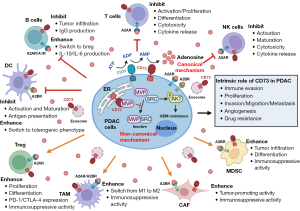
In addition to its immune inhibitory effects, CD73 is also established to be involved in tumorigenesis, proliferation, adhesion/migration, angiogenesis and metastasis (12). In the published study by Yu et al. (4), CD73 was characterized as a novel mechanism for gemcitabine resistance in PDAC. Authors demonstrated that CD73 is highly expressed in PDAC tumor cells and is associated with poor prognosis following the gemcitabine treatment. Long-term treatment of gemcitabine leads to an elevated expression of CD73 in PDAC cells, suggesting that CD73 may play a key role in developing the secondary resistance to gemcitabine. Thus, this study has identified CD73 as a potential target for overcoming gemcitabine resistance in PDAC.
Three isoforms of CD73 have been reported in human cells and were found to play different or opposite biological roles (28,29). Among these three isoforms, the short splice variant of CD73 (CD73S) was reported to be expressed in cirrhotic livers and hepatocellular carcinoma and, by binding to the full-length isoform of CD73 (CD73L), to promote the degradation of CD73L (29). Therefore, Yu et al. (4) investigated the activities of various CD73 isoforms, respectively, in order to identify the optimal targets for anti-CD73 therapies in PDAC. Interestingly, the authors found that the two major isoforms of CD73, CD73L and CD73S, displayed a similar effect in mediating the gemcitabine resistance in PDAC. In addition, inhibiting one of these two isoforms would lead to the suppression of the other one in PDAC cells. Thus, their findings suggest that the anti-CD73 therapy can be designed to target both isoforms, regardless of the selectivity for or relative expression levels of each different CD73 isoform in PDAC.
As described above, CD73 plays impotent roles in tumor progression and tumor immune evasion through activating adenosine signaling (30). In the study by Yu et al. (4), the authors demonstrated that CD73 is also located on the organelle membrane such as the endoplasmic reticulum (ER), where it competitively interacts with the main vault protein (MVP) to activate SRC Proto-Oncogene, Non-Receptor Tyrosine Kinase (SRC) kinase and thereby mediates the gemcitabine resistance by inducing the activation of AKT Serine/Threonine Kinase (AKT) pathway in PDAC tumor cells (Figure 1). Thus, Yu et al. (4) identified a novel non-canonical function of CD73 involved in the mechanism of resistance to gemcitabine-based chemotherapy. This novel function of CD74 is independent of its nucleotidase activity.
To date, multiple therapeutic agents that inhibit CD73, including mAbs that block membrane-bound CD73 and small molecules that inhibit its nucleotidase activity, have been developed and are currently evaluated in the early phase of clinical trials in cancer patients including PDAC patients (6,31). However, the results on treating PDAC patients in some of these clinical trials have been disappointing (NCT03954704, NCT04262388, and NCT04262375). The study published by Yu et al. (4) thus raised a question on whether the pro-tumoral activities of CD73, including both its nucleotidase activity and its ER associated activity, have been adequately targeted. Therefore, the authors suggest that inhibition of CD73 expression may be a better targeting strategy, which would synchronously inhibit both membrane-bound and ER-located, as well as the nucleotidase-dependent and independent activities of CD73 in PDAC cells.
Yu et al. (4) demonstrated that troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist currently used in the clinic to treat diabetic mellitus, dramatically suppresses the expression of both CD73L and CD73S isoforms and sensitizes PDAC to the gemcitabine-based chemotherapy. Accordingly, troglitazone may be repurposed as a supplemental anti-CD73 strategy in combination with gemcitabine-based chemotherapy for PDAC treatment. This novel treatment strategy warrants further investigation in clinical trials.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grants 82030092, 81720108028 for JH, and 82272799 for AC).
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Associate Editor, Min Li, PhD (The University of Oklahoma Health Sciences Center, Stanton L. Young Biomedical Research Center, Oklahoma City, OK, USA) and the Editor-in-Chief, Lei Zheng, MD, PhD (Departments of Oncology and Surgery, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-2022-2/coif). The author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Zeng S, Pöttler M, Lan B, et al. Chemoresistance in Pancreatic Cancer. Int J Mol Sci 2019;20:4504. [Crossref] [PubMed]
- Amrutkar M, Gladhaug IP. Pancreatic Cancer Chemoresistance to Gemcitabine. Cancers (Basel) 2017;9:157. [Crossref] [PubMed]
- Yu X, Liu W, Wang Z, et al. CD73 induces gemcitabine resistance in pancreatic ductal adenocarcinoma: A promising target with non-canonical mechanisms. Cancer Lett 2021;519:289-303. [Crossref] [PubMed]
- Chen S, Wainwright DA, Wu JD, et al. CD73: an emerging checkpoint for cancer immunotherapy. Immunotherapy 2019;11:983-97. [Crossref] [PubMed]
- Battastini AMO, Figueiró F, Leal DBR, et al. CD39 and CD73 as Promising Therapeutic Targets: What Could Be the Limitations? Front Pharmacol 2021;12:633603. [Crossref] [PubMed]
- Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51-72. [Crossref] [PubMed]
- Leone RD, Emens LA. Targeting adenosine for cancer immunotherapy. J Immunother Cancer 2018;6:57. [Crossref] [PubMed]
- Roh M, Wainwright DA, Wu JD, et al. Targeting CD73 to augment cancer immunotherapy. Curr Opin Pharmacol 2020;53:66-76. [Crossref] [PubMed]
- Allard B, Longhi MS, Robson SC, et al. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev 2017;276:121-44. [Crossref] [PubMed]
- Vigano S, Alatzoglou D, Irving M, et al. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front Immunol 2019;10:925. [Crossref] [PubMed]
- Chen Q, Pu N, Yin H, et al. CD73 acts as a prognostic biomarker and promotes progression and immune escape in pancreatic cancer. J Cell Mol Med 2020;24:8674-86. [Crossref] [PubMed]
- Yang H, Yao F, Davis PF, et al. CD73, Tumor Plasticity and Immune Evasion in Solid Cancers. Cancers (Basel) 2021;13:177. [Crossref] [PubMed]
- Huang S, Apasov S, Koshiba M, et al. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 1997;90:1600-10. [Crossref] [PubMed]
- Bono MR, Fernández D, Flores-Santibáñez F, et al. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett 2015;589:3454-60. [Crossref] [PubMed]
- Chambers AM, Wang J, Lupo KB, et al. Adenosinergic Signaling Alters Natural Killer Cell Functional Responses. Front Immunol 2018;9:2533. [Crossref] [PubMed]
- Häusler SF, Montalbán del Barrio I, Strohschein J, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother 2011;60:1405-18. [Crossref] [PubMed]
- Ohta A, Kini R, Ohta A, et al. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol 2012;3:190. [Crossref] [PubMed]
- Di Gennaro P, Gerlini G, Caporale R, et al. T regulatory cells mediate immunosuppresion by adenosine in peripheral blood, sentinel lymph node and TILs from melanoma patients. Cancer Lett 2018;417:124-30. [Crossref] [PubMed]
- Ryzhov S, Novitskiy SV, Goldstein AE, et al. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol 2011;187:6120-9. [Crossref] [PubMed]
- Iannone R, Miele L, Maiolino P, et al. Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia 2013;15:1400-9. [Crossref] [PubMed]
- Stagg J, Divisekera U, McLaughlin N, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 2010;107:1547-52. [Crossref] [PubMed]
- Hay CM, Sult E, Huang Q, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology 2016;5:e1208875. [Crossref] [PubMed]
- Allard B, Pommey S, Smyth MJ, et al. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res 2013;19:5626-35. [Crossref] [PubMed]
- Young A, Ngiow SF, Barkauskas DS, et al. Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses. Cancer Cell 2016;30:391-403. [Crossref] [PubMed]
- Blocking CD73 Can Shrink Pancreatic Tumors. Cancer Discov 2021;11:OF4.
- Herbst RS, Majem M, Barlesi F, et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 2022;40:3383-93. [Crossref] [PubMed]
- Scotlandi K, Zuntini M, Manara MC, et al. CD99 isoforms dictate opposite functions in tumour malignancy and metastases by activating or repressing c-Src kinase activity. Oncogene 2007;26:6604-18. [Crossref] [PubMed]
- Snider NT, Altshuler PJ, Wan S, et al. Alternative splicing of human NT5E in cirrhosis and hepatocellular carcinoma produces a negative regulator of ecto-5'-nucleotidase (CD73). Mol Biol Cell 2014;25:4024-33. [Crossref] [PubMed]
- de Araújo JB, Kerkhoff VV, de Oliveira Maciel SFV, et al. Targeting the purinergic pathway in breast cancer and its therapeutic applications. Purinergic Signal 2021;17:179-200. [Crossref] [PubMed]
- Nocentini A, Capasso C, Supuran CT. Small-molecule CD73 inhibitors for the immunotherapy of cancer: a patent and literature review (2017-present). Expert Opin Ther Pat 2021;31:867-76. [Crossref] [PubMed]
Cite this article as: Chang A, Hao J. A tumor intrinsic role of CD73 in pancreatic adenocarcinoma. Ann Pancreat Cancer 2022;5:9.


