Pancreatic metastasis of a primary osteosarcoma with genomic profiling analysis: case report and review of the literature
Highlight box
Key findings
• Our case report highlights a rare presentation of a patient with pancreatic metastasis of osteosarcoma.
What is known and what is new?
• Osteosarcoma metastasis to the pancreas is unusual. We summarize the existing literature of this uncommon presentation.
• We performed next generation sequencing on the pancreatic metastasis which identified multiple copy number alterations in TP53, PTEN, RNF43, and SMAD4.
What is the implication, and what should change now?
• Improvement in therapies for osteosarcoma is leading to more unusual sites of metastasis which should affect surveillance strategies and intervals.
• Further study is needed on optimal therapies for management of pancreatic metastasis of osteosarcoma.
• Next generation sequencing could provide insight into genetic predisposition for pancreatic metastasis of osteosarcoma. This could eventually impact surveillance and management strategies.
Introduction
Osteosarcoma is the most common primary bone malignancy. It demonstrates bimodal age distribution with peak incidence in early adolescence and in adults over the age of 65 (1). Considered uncommon in adults, it represents the 8th most common cause of childhood and adolescent cancer, accounting for 2.4% of all malignancies in children (2). In younger ages, osteosarcoma coincides with pubertal growth spurts as evidenced by increased incidence in adolescents ages (2). The second peak over the age of 65 is typically secondary to other bony pathology such as Paget’s or previous irradiation (3). Males are more commonly affected than females. Black and Hispanic children are more commonly afflicted than Caucasians in childhood whereas the condition is more common in non-Hispanic white patients in adult-onset disease. Primary tumors are most commonly seen near the metaphyseal growth plates of long bones in the extremities such as the femur, tibia and the humerus (2). Most osteosarcoma are sporadic although genetic predisposition, particularly in children, is common with germ-line mutations commonly identified in TP53, RB1 and RECQL4 (4,5). Unfortunately, 25% of patients have metastatic disease at time of diagnosis (4). In fact, 80% of patients are considered to have pulmonary micrometastasis (6). The lungs represent the most common site of metastatic disease (98%) with other sites including the skeletal system (37%), pleura (33%), cardiac system (20%), renal and hepatic (17%), and mediastinum (11%) (7). Metastasis of osteosarcoma to the pancreas is very uncommon. Due to better treatment modalities with increased survival rates, metastasis to more unusual sites are being increasingly identified (8). In this case report, we will discuss a case of a pancreatic metastasis from primary osteosarcoma with molecular analysis using next generation sequencing and review the existing literature of this rare presentation. We present the following case in accordance with the CARE reporting checklist (available at https://apc.amegroups.com/article/view/10.21037/apc-22-5/rc).
Case presentation
An 18-year-old previously healthy male presented with complaints of progressively worsening left knee pain. Magnetic resonance imaging (MRI) and biopsy confirmed a high grade, poorly differentiated, osteosarcoma of the distal left femoral diaphysis, metaphysis, and epiphysis (lateral condyle) with extension into the adjacent posterior and lateral thigh soft tissues measuring 7.1 cm × 6.6 cm × 10.4 cm. Staging demonstrated multiple scattered nodules throughout the lungs bilaterally measuring up to 5 mm suggesting micrometastasis. The patient was started on chemotherapy per the AOST0331 protocol, which included cisplatin, doxorubicin, and methotrexate. After 4 rounds of chemotherapy, the patient underwent limb salvage surgery with total knee replacement. Pathology demonstrated high grade osteoblastic osteosarcoma with moderate tumor necrosis (60–70%). One year later, the patient developed pulmonary macrometastatic disease and underwent thoracotomy and metasectomy and was started on adjuvant Regorafenib. He subsequently underwent radiation therapy of 2 contiguous lesions of the left upper lobe.
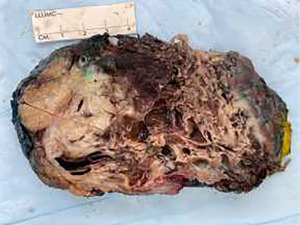
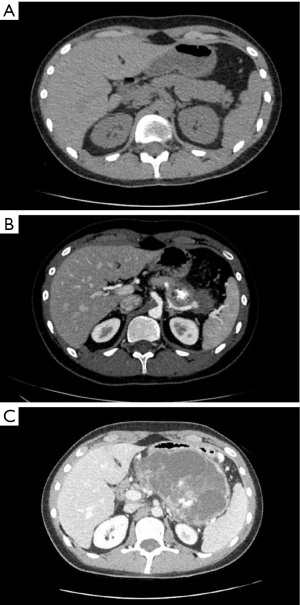
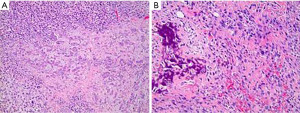
Surveillance imaging on December 2020 demonstrated no signs of pancreatic involvement (Figure 1A), however, subsequent surveillance imaging done on May 2021 (two years after diagnosis) demonstrated an increase in size of multiple pulmonary metastases in addition to a new finding of a partially calcified mixed solid/cystic pancreatic lesion within the pancreatic tail and adjacent regional stranding, concerning for metastatic disease or focal pancreatitis (Figure 1B). Patient denied abdominal pain. Lipase was elevated at 782 U/L [>3 upper limit of normal (ULN)]. Liver enzymes were normal. Due to concern for possible drug induced pancreatitis, Regorafenib was held. Follow-up magnetic resonance cholangiopancreatography (MRCP) in June 2021 demonstrated an enlarging mixed cystic and solid mass lesion, appearing to originate from the superior aspect of the pancreatic body/tail measuring up to 6.8 cm. The patient underwent endoscopic ultrasound (EUS) with fine needle aspiration (FNA). EUS confirmed a large (6.4 cm × 6.3 cm) mixed solid cystic exophytic mass arising from the pancreatic tail. The mass itself was seen to have thin septations and was multicystic with no clear communication with the main pancreatic duct. The pancreas was otherwise normal. FNA was completed with 8 cc of blood-tinged amber fluid. Fluid was sent for cytology, chemistry and next generation sequencing (PancreaSeq®, University of Pittsburgh Molecular Lab). Carcinoembryonic antigen (CEA) and amylase were within normal limits. Cytology demonstrated cystic contents consisting of a few strips and clusters of bland ductal type epithelial cells and scattered degenerating cells (likely histiocytes and epithelial cells). No malignancy, including osteosarcoma was noted. PancreaSeq identified multiple genomic alterations including PTEN, TP53, RNF43, and SMAD4 copy number alterations, however no gene mutations (such as point mutations, insertions, or deletions) were noted. Patient was discharged with no complications. A subsequent computed tomography (CT) abdomen with contrast was completed in July 2021 to re-assess the pancreatic lesion demonstrated interval enlargement of both pancreatic mixed cystic/solid mass (Figure 1C) and pulmonary nodules, as well as the development of a new hypoenhancing lesion in the left inferior renal pole, concerning for metastasis. Given interval enlargement of cystic/solid mass concerning for metastasis, leading to an almost complete inability of the patient to tolerate oral intake due to mass effect on the stomach, the patient was referred to surgical oncology for palliative measures and underwent en-bloc resection of the mass, distal subtotal gastrectomy, en-bloc spleen-preserving distal pancreatectomy, en-bloc resection of transverse mesocolon and Roux-en-Y gastrojejunostomy reconstruction. The 15 cm mass (Figure 2) was found to be occupying the entire lesser sac and fixed to the mid and distal pancreas, the entire posterior wall of the stomach, the transverse mesocolon, and the splenic vasculature distally. Pathology from mass resection demonstrated metastatic high-grade osteosarcoma with positive perineural and perivascular invasion (Figure 3). Due to progressive disease, the patient later elected to proceed with palliative care.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Pancreatic metastasis of osteosarcoma remains uncommon however with improving treatment modalities for osteosarcoma metastasis to uncommon sites are being increasingly recognized. Based on our literature review, there have been only 20 documented cases of pancreatic metastasis from osteosarcoma (Table 1).
Table 1
| Age/gender | Location of original OS | Time between diagnosis of OS and pancreatic metastasis | Location of pancreatic Mets | Size of pancreatic Mets | Description of pancreatic Mets | EUS-FNA | Tx of metastasis |
|---|---|---|---|---|---|---|---|
| 35 F (7) | Distal right femur | 5 years | Body | 0.3×0.3×0.3 cm | Solid tan/white calcified | No | Resection with distal pancreatectomy with splenectomy |
| 25 F (8) | Right femur | 5 years | Head | 10×4 cm and 5×3×3 cm (two masses in continuity—dumbbell shape) | – | No; resection showed malignant osteoid tissue | Pancreato-duodenectomy |
| 53 F (9) | Left distal femur | 4 years | Genu, body, and tail | 11×10 cm (after drainage several days prior) | Cystic/solid | Yes; FNA resulted in malignant cells with numerous multi-nucleated giant cells. Tumor was highly cellular with 17 mitoses per 10 HPF | Needle aspiration of cystic portion for symptom control |
| 14 M (10) | Vertebral: T7-S1 | 6 years | Head | 8×7.5 cm | Solid with calcifications and necrotic areas | No; Tru-cut biopsy | Deceased prior to treatment |
| 15 F (11) | Distal left femur | 4 years | Tail | 15×15×12 cm, however only small solid portion invaded the pancreas | Cystic lesion with solid component invading the pancreas | Yes; showed pancreatic cells and plemorphic tumoral cells surrounded by osteoid matrix | Partial resection of pancreatic tail. Family refused adjuvant CTx |
| 13 F (12) | Left fibula | 14 months | Head | 6×4×3 cm | Solid | No; resection showed rapidly proliferating osteoblasts with giant and atypical nuclei | Pancreato-duodenectomy |
| 44 F (13) | Right fibular head | 3 years | Tail | 13 mm | Heterogenous solid | Yes; pancreatic islet tissue was also detected in tumor suggesting invasion of tumor into pancreatic body | Laparoscopic splenic-preserving pancreatic tail resection with post-operative CTx |
| 33 M (14) | Right maxillary sinus | 3 years | Body-tail junction | 25 mm | Solid with thin central calcifications | Yes; showed poorly differentiated area consisting of small sized round fusiform cells and focal area of osteoid deposited in a fine lace-like pattern, as well as relatively well differentiated area with cartilage | Patient was undergoing CTx at the time the case report was written |
| 18 M (15) | Proximal left tibia | 4 years | Head | 4.3×4.5 cm | Cystic-solid mass | Yes; amorphous pink-colored osteoid structures with pleomorphic multinucleated sarcomatoid cells | Pancreato-duodenectomy followed by CTx |
| 66 F (16) | Femur | 3 years | – | – | – | No | – |
| 9 F (16) | Femur | 1.5 years | – | – | – | No | CTx |
| 14 F (16) | Femur | 11 years | – | – | – | No | Pancreato-duodenectomy |
| 57 M (17) | Femur | 3 years | – | – | – | – | – |
| 52 M (18) | Maxillary sinus | 2 years | – | – | – | Yes | – |
| 58 F (19) | Tibia | 7.3 years | Head | 3.5 cm | – | – | Radiation and CTx |
| 57 F (19) | Femur | 1.25 years prior to diagnosis of osteosarcoma | Tail | 2.7 cm | Solid | No | Distal pancreatectomy followed by CTx |
| 32 F (19) | Skull | 5.4 years | Tail | 19 cm | – | No | Distal pancreatectomy |
| 28 F (19) | Femur | 1.7 years | Body | 4 cm | – | No | Distal pancreatectomy |
| 63 F (20) | Left distal femur | – | Head | 3.6×2.6 cm | Cystic | Yes; FNA showed metastatic malignant tumor with highly pleomorphic atypical tumor cells | None |
| 38 F (21) | – | 3.6 years | Head | 5.7 cm | Cystic mass with calcifications | – | – |
| 19 F (22) | Distal femur | 5.5 years | – | 5 cm | – | No | Pancreato-duodenectomy followed by adjuvant CTx |
| 47 F (23) | Distal femur | 3.3 years | Head | 7 cm | Solid | – | Radiation therapy |
| 42 F (23) | Left distal femur | – | Tail | – | Solid | – | Radiation therapy |
OS, osteosarcoma; Mets, metastasis; EUS, endoscopic ultrasound; FNA, fine needle aspiration; Tx, treatment; F, female; M, male; HPF, high-power field; CTx, chemotherapy.
Aside from our case, there has been no documented genetic analysis of pancreatic metastasis from osteosarcoma. It has been shown that osteosarcoma with positive PTEN expression has been associated with high differentiation on histology, less chance of metastasis, and higher overall 5-year survival rate (24). It is thought that PTEN expression can inhibit osteosarcoma cell proliferation and migration (25). USP17 upregulation, through stabilization of SMAD4, has been thought to promote cell proliferation, epithelial-mesenchymal transition, and metastasis in osteosarcoma (26). Activation of HIF-1α and AP-1 genetic pathways have revealed a possible increase in metastatic potential (27). In addition, APEX1 expression in osteosarcoma has also been found to be an independent predictor of local recurrence and metastasis, owing to chemoresistance and radioresistance (28). Genetic markers that increase the risk of osteosarcoma development include germline variants in TP53, RB1, and RECQL4 genes (5). ATRX germline variant has also been thought to be associated with an increase in the risk of osteosarcoma development (5). In our patient, PancreaSeq identified multiple copy number alterations in TP53, PTEN, RNF43, and SMAD4. As noted above, alterations in PTEN and SMAD4 have been associated with increased risk of metastasis.
In our case, pancreatic metastasis was identified 2 years after diagnosis which is consistent with the existing case reports. In addition, despite an elevated lipase, our patient was asymptomatic which is consistent with the published cases (7). It is worthy to note that most documented pancreatic involvement in osteosarcoma represents solitary solid lesions (9). In our particular case, the patient’s pancreatic metastasis was found to be a single metastatic cystic/solid lesion. There are very few cases in the literature of pancreatic cystic or cystic/solid metastasis from osteosarcoma. The pathophysiology of cystic metastasis is unknown. Current theories include necrotic degeneration of a solid mass or pancreatitis from the metastasis leading to fluid collections (9). Necrotic degeneration of solid masses is seen more commonly in neuroendocrine tumors, ductal adenocarcinomas, and acinar cell carcinoma (9). Pseudocyst formation secondary to metastasis-induced acute pancreatitis could be possible in the case of our patient given his elevation in lipase. Though it is important to note that regorafenib can increase serum lipase and even cause pancreatitis (29). Diagnosis of pancreatic metastasis can be confirmed with EUS-FNA although if inconclusive and the index of suspicion remains high, surgery may be warranted. Management of pancreatic metastasis of osteosarcoma involves a combination of surgery, radiation and chemotherapy. Surgical resection is considered an important modality of local control of metastatic sites although the benefit in osteosarcoma metastasis to the pancreas appears unproven (4). Ideal candidates for surgical resection should have isolated resectable pancreatic metastasis after complete staging or limited other sites also amenable to resection. Typical pancreatic resection approaches such as pancreaticoduodenectomy or distal pancreatectomy are favored over enucleation or central pancreatectomies owing to higher local recurrence rates despite less morbidity (12). Osteosarcoma is not a radiosensitive malignancy and thus radiation is typically offered as adjuvant therapy for consolidation treatment or as definitive management in unresectable disease.
In summary, as treatment advances for osteosarcoma, more unusual sites of metastasis will occur. Genetic profiling of osteosarcomas may help identify if there are any particular genetic alterations that would give a predilection to non-pulmonary metastasis, as in the case of our patient. This could allow for patient centered surveillance strategies for a select group of individuals and allow for timely diagnosis and treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apc.amegroups.com/article/view/10.21037/apc-22-5/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apc.amegroups.com/article/view/10.21037/apc-22-5/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009;115:1531-43. [Crossref] [PubMed]
- Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 2009;152:3-13. [Crossref] [PubMed]
- Huvos AG. Osteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 years. Cancer 1986;57:1442-9. [Crossref] [PubMed]
- Eaton BR, Schwarz R, Vatner R, et al. Osteosarcoma. Pediatr Blood Cancer 2021;68:e28352. [Crossref] [PubMed]
- Masliah-Planchon J, Lévy D, Héron D, et al. Does ATRX germline variation predispose to osteosarcoma? Three additional cases of osteosarcoma in two ATR-X syndrome patients. Eur J Hum Genet 2018;26:1217-21. [Crossref] [PubMed]
- Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res 2009;152:239-62. [Crossref] [PubMed]
- Cirotski D, Panicker J. Parosteal Osteosarcoma with Pancreatic Metastasis and Multiple Relapses: A Case Report and Review of the Literature. Case Rep Oncol 2021;2021:983-8. [Crossref] [PubMed]
- Aarvold A, Bann S, Giblin V, et al. Osteosarcoma metastasising to the duodenum and pancreas. J Bone Joint Surg Br 2007;89:542-4. [Crossref] [PubMed]
- Akpinar B, Obuch J, Fukami N, et al. Unusual presentation of a pancreatic cyst resulting from osteosarcoma metastasis. World J Gastroenterol 2015;21:8452-7. [Crossref] [PubMed]
- Avcu S, Akdeniz H, Arslan H, et al. A case of primary vertebral osteosarcoma metastasizing to pancreas. JOP 2009;10:438-40. [PubMed]
- Rejin K, Aykan OA, Omer G, et al. Intra-abdominal metastasis in osteosarcoma: survey and literature review. Pediatr Hematol Oncol 2011;28:609-15. [Crossref] [PubMed]
- Lasithiotakis K, Petrakis I, Georgiadis G, et al. Pancreatic resection for metastasis to the pancreas from colon and lung cancer, and osteosarcoma. JOP 2010;11:593-6. [PubMed]
- Toyama H, Asari S, Goto T, et al. A Case of Pancreatic Metastasis of Osteosarcoma Resected Using Laparoscopic Spleen Preserving Distal Pancreatectomy. Gan To Kagaku Ryoho 2016;43:1988-90. [PubMed]
- Araújo JC, Carvalho Junior JA, Monges G, et al. Pancreatic and lymph node metastases from maxillary osteosarcoma diagnosed by endoscopic ultrasound-guided fine needle aspiration. Endoscopy 2012;44 Suppl 2 UCTN:E151-2.
- Jin P, Wang W, Su H, et al. Osteosarcoma metastasizing to pancreas confirmed by endoscopic ultrasound-guided fine-needle aspiration. Endoscopy 2014;46 Suppl 1 UCTN:E109-10.
- Bertucci F, Araujo J, Giovannini M. Pancreatic metastasis from osteosarcoma and Ewing sarcoma: literature review. Scand J Gastroenterol 2013;48:4-8. [Crossref] [PubMed]
- Kim SJ, Choi JA, Lee SH, et al. Imaging findings of extrapulmonary metastases of osteosarcoma. Clin Imaging 2004;28:291-300. [Crossref] [PubMed]
- Khan AS, Crowe DR, Trevino JM, et al. Multiple metastases to the pancreas from primary maxillary osteosarcoma: diagnosis with EUS-guided FNA. Gastrointest Endosc 2011;73:1320-2. [Crossref] [PubMed]
- Lee M, Song JS, Hong SM, et al. Sarcoma metastasis to the pancreas: experience at a single institution. J Pathol Transl Med 2020;54:220-7. [Crossref] [PubMed]
- Gonakoti S, Dantey K, Kochhar GS. Not All Pancreatic Masses Are Pancreatic Adenocarcinoma - A Rare Presentation of Pancreatic and Duodenal Metastases From Primary Osteosarcoma. Am J Gastroenterol 2021;116:S720-S721. [Crossref]
- Shi HY, Zhao XS, Miao F. Metastases to the Pancreas: Computed Tomography Imaging Spectrum and Clinical Features: A Retrospective Study of 18 Patients With 36 Metastases. Medicine (Baltimore) 2015;94:e913. [Crossref] [PubMed]
- Huddy JR, Sodergren MH, Deguara J, et al. Pancreaticoduodenectomy for the Management of Pancreatic or Duodenal Metastases from Primary Sarcoma. Anticancer Res 2018;38:4041-6. [Crossref] [PubMed]
- Yoshida K, Nakamura T, Hagi T, et al. Acute pancreatitis due to pancreatic metastasis of osteosarcoma: a report of two cases. Ann Joint 2019;4:41. [Crossref]
- Zhou J, Xiao X, Wang W, et al. Association between PTEN and clinical-pathological features of osteosarcoma. Biosci Rep 2019;39:BSR20190954. [Crossref] [PubMed]
- Liu Q, Geng P, Shi L, et al. miR-29 promotes osteosarcoma cell proliferation and migration by targeting PTEN. Oncol Lett 2019;17:883-90. [PubMed]
- Song C, Liu W, Li J. USP17 is upregulated in osteosarcoma and promotes cell proliferation, metastasis, and epithelial-mesenchymal transition through stabilizing SMAD4. Tumour Biol 2017;39:1010428317717138. [Crossref] [PubMed]
- Maximov VV, Aqeilan RI. Genetic factors conferring metastasis in osteosarcoma. Future Oncol 2016;12:1623-44. [Crossref] [PubMed]
- Yang J, Yang D, Cogdell D, et al. APEX1 gene amplification and its protein overexpression in osteosarcoma: correlation with recurrence, metastasis, and survival. Technol Cancer Res Treat 2010;9:161-9. [Crossref] [PubMed]
- Pereira M, Prakash A, Puranik AD, et al. Regorafenib-Associated Acute Pancreatitis Diagnosed on 18F-FDG PET/CT. Clin Nucl Med 2021;46:e256-7. [Crossref] [PubMed]
Cite this article as: Darioosh RP, Singhi A, Reeves ME, Skef W. Pancreatic metastasis of a primary osteosarcoma with genomic profiling analysis: case report and review of the literature. Ann Pancreat Cancer 2023;6:2.